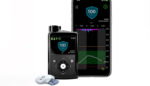
Medtronic (NYSE:MDT) announced today that reimbursement for its CGMs has been expanded or initiated across a number of countries. Earlier this month, the Ontario, Canada provincial government announced a comprehensive reimbursement program for continuous glucose monitoring (CGM) for eligible residents who have type 1 diabetes, adding to a similar program for those with type 1 […]
Integrity Applications changes company name to GlucoTrack
Integrity Applications (Nasdaq:IGAP) announced today that it completed the change of its corporate name to GlucoTrack. Rutherford, New Jersey-based GlucoTrack confirmed the change of its ticker symbol on the Nasdaq capital market to “GCTK,” which was made effective at the commencement of trading today, March 14, 2022. GlucoTrack develops the GlucoTrack non-invasive device and digital […]
FDA clears Fresenius Kabi wireless Agilia Connect infusion system
Fresenius Kabi announced today that it received FDA 510(k) clearance for its wireless Agilia Connect infusion system. Lake Zurich, Illinois-based Fresenius Kabi’s Agilia Connect infusion system includes the Agilia volumetric pump and the Agilia syringe pump with Vigilant software suite-Vigilant Master Med technology. According to a news release, the two pumps are the first to […]
Dexcom wins CE mark for next-gen G7 CGM, will begin European launch within weeks
Dexcom (Nasdaq:DXCM) announced today that it received CE mark approval in Europe for its G7 continuous glucose monitoring (CGM) system. The San Diego–based company has also submitted for an FDA 510(k) for G7 and awaits clearance in the U.S. Dexcom designed its next-generation CGM offering as an all-in-one wearable system. It warms up in 30 […]
Stevanato Group acquires facility set to become manufacturing hub in China
Stevanato Group (NYSE:STVN) announced today that it acquired a facility in Zhangjiagang, China, for a new manufacturing plant. Piombino Dese, Italy-based Stevanato Group expects to begin renovations on the facility in the spring of 2022 as it works to expand in China with a new manufacturing hub for drug containment, drug delivery and diagnostic development. […]
J&J expands it long-acting injectables partnership with Midatech
Midatech Pharma (Nasdaq:MTP) announced today that it extended its R&D collaboration with Johnson & Johnson’s (NYSE:JNJ) Janssen Pharmaceuticals. Cardiff, United Kingdom-based Midatech, a drug delivery technology company focused on the biodelivery and biodistribution of medicines, initially entered into the R&D collaboration with Janssen on July 21, 2020. On June 17, 2021, Midatech confirmed that its […]
Tandem Diabetes warns on some t:slim X2 insulin pumps
Tandem Diabetes Care (Nasdaq:TNDM) issued an urgent field safety notice in Europe for its t:slim X2 insulin pump with Basal-IQ technology. The notice, dated February 2022, alerts users of Tandem’s insulin pump with a software version of 6.3.0.1 that there may be a potential safety risk when using the pump within its normal specifications. Tandem’s […]
Fresenius Kabi recalls sodium acetate injection due to presence of particulate matter
Fresenius Kabi USA announced today that it initiated the voluntary recall of seven lots of its sodium acetate injection. Lake Zurich, Illinois-based Fresenius Kabi recalled the USP, 400 mEq/100 mL (4 mEq/mL), 100 mL fill in a 100 mL vial to the user level due to the presence of particulate matter found in reserve and/or […]
Many infusion pumps are vulnerable to hackers, study says
Palo Alto Network’s Unit 42 released results from a study showing that 75% of infusion pumps observed had known cybersecurity gaps. The results involved crowdsourced data from scans of more than 200,000 infusion pumps on the network of health providers using IoT Security for Healthcare from Palo Alto Networks. Vulnerabilities observed in the study included […]
Koru Medical Systems rises as Q4 earnings beat Street projections
Koru Medical Systems (NSDQ:KRMD) shares ticked up today on fourth-quarter results that topped the consensus forecast. The Chester, New York-based infusion technology developer posted losses of $1.1 million, or 2¢ per share, on sales of $6.5 million for the three months ended Dec. 31, 2021, for a bottom-line slide further into the red on sales […]