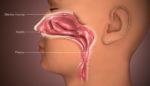
Because of the way they provide protection, nasal vaccines could be the best long-term way to prevent COVID-19 infection, according to experts cited in The New York Times. The Times reported that India-based Bharat Biotech, which has the Covaxin COVID-19 vaccine authorized in India and elsewhere, has an experimental COVID-19 nasal vaccine that may offer […]
BD board approves Embecta diabetes business spinoff
BD (NYSE:BDX) announced today that its board of directors approved the planned spinoff of its diabetes care business, Embecta. Franklin Lakes, N.J.-based BD confirmed in May 2021 that it planned to spin off its diabetes care business as an independent, publicly-traded company during the first half of 2022, and in December 2021, it confirmed the […]
Fractyl Health touts 2-year durability study for diabetes reversal procedure
Fractyl Health today announced that it published two-year durability data from its Revita-1 clinical study in Europe and South America. Lexington, Massachusetts-based Fractyl’s trial included a two-year follow-up from 34 type 2 diabetes patients who received the company’s Revita duodenal mucosal resurfacing (DMR) procedure. According to a news release, the study observed no long-term adverse […]
With FDA clearance ‘imminent’ for Senseonics 180-day CGM, what’s next?
When Dr. Fran Kaufman began her career in the late 1970s, diabetes management consisted of urine testing and animal insulin. At that point, it had not been proven that managing glucose actually mattered, according to Kaufman, and proof didn’t come until 1992 with the conclusion of the DCCT study. “I’ve seen a lot of innovation,” […]
B. Braun receives FDA approval for Florida pharmaceutical manufacturing plant
B. Braun Medical announced today that it received final approval from the FDA for its new pharmaceutical manufacturing plant. Bethlehem, Pennsylvania-based B. Braun’s Daytona Beach, Florida-based plant will produce 0.9% sodium chloride for injection available in B. Braun’s Excel Plus IV bags in 1,000 mL and 500 mL sizes. Get the full story at our […]
Eli Lilly to build $1B manufacturing facility for injectable products, devices in North Carolina
Eli Lilly (NYSE:LLY) announced today that it plans to invest more than $1 billion to create a new manufacturing site in North Carolina. Indianapolis-based Eli Lilly’s new facility, which it expects will create nearly 600 jobs in Concord, North Carolina, will manufacture parenteral (injectable) products and devices while increasing the company’s manufacturing capacity. The company […]
FDA clears Insulet’s next-generation Omnipod 5 wearable insulin delivery pump
Insulet (NSDQ:PODD) announced today that it received FDA clearance for its Omnipod 5 automated insulin delivery system. FDA clearance for the next-generation version of the company’s wearable insulin delivery pump system covers individuals aged six years and older with type 1 diabetes. The platform provides easier glucose management, with no multiple daily injections, no tubes […]
How Bigfoot Biomedical plans to take diabetes management forward
For close to 20 years, Jeffrey Brewer has focused on finding ways to support insulin delivery and manage diabetes. Initially an entrepreneur, Brewer got involved with the Juvenile Diabetes Research Foundation (JDRF) and the Artificial Pancreas Project for developing a closed-loop insulin delivery system after his son was diagnosed with type 1 diabetes. After spending […]
Stevanato Group extends licensing agreement with Haselmeier for pen injector tech
Stevanato Group (NYSE:STVN) announced today that it signed an extension to a licensing agreement with Haselmeier over its pen injector technology. Piombino Dese, Italy-based Stevanato Group’s license extension gives the company exclusivity to offer the Axis-D pen injector technology in support of a broader range of drugs for its biopharma customers beyond diabetes, including areas […]
Glytec enters insulin management partnership with Nebraska Medicine
Glytec announced today that it entered into a strategic partnership with Nebraska Medicine to advance patient safety and innovation in insulin management. Under the agreement, Nebraska Medicine will implement Glytec’s eGlycemic Management System (eGMS) across all 800 beds at its two hospitals: the Nebraska Medical Center and Bellevue Medical Center. The agreement also includes collaboration […]