Researchers from Duke University have developed a biopolymer that could provide weeks of glucose control for patients with diabetes – in a single injection.
The controlled-release treatment lasted for weeks in primates, according to a study published in Nature Biomedical Engineering. The biomedical engineers suggested that this new, injectable solution could replace daily or weekly insulin shots for people with Type II diabetes.
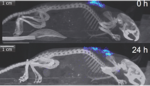
The team at Duke created a technology that combines a signaling molecule, glucagon-like peptide-1, with a heat-sensitive polypeptide in an injectable solution. After it’s administered, the solution reacts with body heat and forms a biodegradable depot that slowly deposits the drug as it dissolves.
The therapy regulated blood glucose in animal models up to 3 times longer than conventional treatment methods.
“Although we’ve pursued this method in the past, Kelli Luginbuhl, a grad student in my lab, systematically worked to vary the design of the delivery biopolymer at the molecular level and found a sweet spot that maximized the duration of the drug’s delivery from a single injection,” senior author Ashutosh Chilkoti said in prepared remarks. “By doing so, we managed to triple the duration of this short-acting drug for type 2 diabetes, outperforming other competing designs.”
Chilkoti is a scientific advisor at PhaseBio Pharmaceuticals, which licensed the glucose therapy tech from the team at Duke.
The injectable glucose management therapy regulated blood sugar levels in mice for 10 days and in rhesus monkeys for more than 14 days after a single injection, according to the team. The researchers also reported that the drug released at a constant rate for the duration of the preclinical study.
“What’s exciting about this work was our ability to demonstrate that the drug could last over 2 weeks in non-human primates,” co-author Luginbuhl said. “Because our metabolism is slower than monkeys and mice, the treatment should theoretically last even longer in humans, so our hope is that this will be the 1st bi-weekly or once-a-month formulation for people with Type II diabetes.”
Next, the team hopes to evaluate its biopolymer in other animal models and assess the immunological aspects of repeat injections.

