BD (NYSE:BDX) announced today that orders for its needles and syringes hit 2 billion amid global COVID-19 vaccination efforts. Franklin Lakes, N.J.-based BD’s latest milestone comes less than six months after the company surpassed 1 million orders for vaccine delivery devices. The order total encompasses commitments to governments all over the globe, including the U.S., […]
Business/Financial News
Carbon Health acquires Steady Health and its diabetes platform
Carbon Health announced today that it acquired Steady Health and its platform for using continuous glucose monitoring to personalize diabetes care. San Francisco–based Carbon Health said in a news release that the acquisition accelerates its rollout of a new primary care model designed to integrate its clinic footprint with virtual and hardware-enabled capabilities to create […]
GM Instruments, Sensonics partner on nasal and oral measurement devices
GM Instruments announced today that it partnered with Sensonics to develop devices for the quantitative assessment of nasal and oral function. The companies tout the first-of-its-kind partnership as a bringing together of sensory and engineering expertise for evaluating and treating nasal and oral diseases. Additionally, the companies expect the partnership to establish the influences of […]
FDA accepts resubmitted NDA for Xipere from Clearside Biomedical, Bausch Health
Bausch Health (NYSE:BHC) and Clearside Biomedical (NSDQ:CLSD) announced today that the FDA accepted their resubmitted new drug application for Xipere. Clearside Biomedical designed the investigational Xipere triamcinolone acetonide suprachoroidal injectable suspension therapy with a proposed indication for treating macular edema associated with uveitis. Xipere works with Clearside’s proprietary SCS Microinjector to access the back of […]
Lyra inks $135M partnership to expand chronic rhinosinusitis treatment in Asia
Lyra Therapeutics (NSDQ:LYRA) announced today that it entered into a strategic partnership with LianBio for its LYR-210 treatment for chronic rhinosinusitis. Watertown, Mass.–based Lyra, which develops the LYR-210 therapeutic to be locally delivered by its proprietary XTreo platform, could receive up to $135 million in total payments through the strategic partnership and exclusive license agreement […]
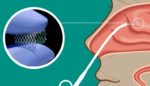
Noveome Biotherapeutics inks licensing deal for SipNose drug delivery device
Noveome Biotherapeutics announced that it entered a commercial license agreement to use SipNose’s proprietary drug delivery device. Yokneam, Israel–based SipNose develops its intranasal Cribriform Targeted Device (CT6), which, under the agreement, was licensed to Noveome to deliver the Pittsburgh-based company’s ST266 biologic to the central nervous systems, according to a news release. CT6 allows aerosolized […]
Phillips-Medisize unveils Aria smart autoinjector
Phillips-Medisize today unveiled the Aria smart autoinjector platform designed to elevate patient care and reduce environmental impact. Hudson, Wis.–based Phillips-Medisize, a Molex company, said in a news release that the Aria platform introduces a small, simple smart injection device with a reusable electronic drive unit and single-use, disposable cassettes. The company designed the Aria autoinjector […]
Medtronic wins CE mark for smart insulin pen, Guardian 4 sensor
Medtronic (NYSE:MDT) announced today that it received CE mark approvals for its InPen smart insulin pen and its Guardian 4 sensor. Fridley, Minn.-based Medtronic’s new European approvals cover expanded functionality of the InPen for multiple daily injections (MDI), along with approval for the Guardian 4 sensor that requires no fingersticks for calibration or diabetes treatment […]
Retractable Technologies wins $27M federal contract
Retractable Technologies (NYSE:RVP) announced that it has received a government contract worth more than $27.3 million for its syringes. The new contract is the third multi-million-dollar deal that the U.S. Department of Health & Human Services (HHS) has awarded to the Little Elm, Texas-based company since February 2020. The company landed an $83.8 million contract with […]
Nanoform, Celanese partner to develop nanoparticle-enabled drug delivery
Nanoform announced today that it partnered with Celanese to explore the use of their technologies in nanoparticle-enabled drug delivery. Helsinki-based Nanoform said the goal of the collaboration is to assess the combination of its nanoparticle platform technologies with Celanese’s VitalDose EVA copolymer delivery technology for drug-eluting implants, according to a news release. Both companies intend […]