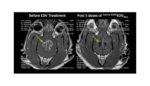
EnGeneIC said today that the FDA granted orphan drug designation to the company’s targeted EDV nanocells for the treatment of glioblastoma multiforme. The clinical stage biopharmaceutical company is developing bacterially-derived EDV nanocells as a nanoparticle, siRNA and miRNA delivery platform designed to target and kill tumor cells, while triggering the immune system’s natural anti-tumor response. […]
Food & Drug Administration (FDA)
Aerie resubmits NDA for Rhopressa eye drops
Aerie Pharmaceuticals (NSDQ:AERI) said today that it resubmit a New Drug Application for the company’s Rhopressa eye drops. The Irvine, Calif.-based company’s NDA was withdrawn in October last year, after a contract manufacturer was not prepared for its pre-approval inspection. Aerie’s Rhopressa eye drops specifically target the eye’s trabecular network – the diseased tissue responsible for […]
Trump calls for speedier approval process, while Sanders takes aim at drug prices
In his 1st address to Congress, President Donald Trump called for speeding up drug approvals within the FDA. Earlier that day, Sen. Bernie Sanders (I-Vermont) proposed a bill to allow the importation of prescription drugs from Canada in the hopes of lowering drug prices. The new administration has been critical of drug prices, with Trump saying that […]
FDA asks Titan for more info before clinical trial of ropinirole implant
Titan Pharmaceuticals (NSDQ:TTNP) said today that the FDA put a hold on the clinical trial of its ropinirole implant and told the company to submit more information to the federal watchdog. After it completed an initial review of the implant’s Investigational New Drug application, the FDA asked Titan for final release test data on its ropinirole […]
OncoSec wins fast track designation for electroporation combo therapy
OncoSec Medical (NSDQ:ONCS) said today that it won Fast Track Designation from the FDA for its ImmunoPulse IL-12 electroporation gene therapy for the treatment of metastatic melanoma. The biotech company’s electroporation device is designed to locally deliver DNA-based interleukin-12 to stimulate the immune system and fight off cancer cells. The ImmunoPulse device delivers a sequence […]
FDA accepts AcelRx’s NDA for Dsuvia pain reliever
AcelRx Pharmaceuticals (NSDQ:ACRX) said today that the FDA accepted the company’s New Drug Application for its Dsuvia pain relief therapy. The federal watchdog set a target decision date for October 12 this year. The company’s Dsuvia candidate is composed of 30 sufentanil tablets delivered sublingually using a disposable, pre-filled single-dose applicator to patients with moderate-to-severe […]
Intersect ENT wins FDA nod for Propel Contour steroid-releasing implant
Intersect ENT (NSDQ:XENT) said today that it won FDA approval for its Propel Contour steroid-releasing implant for the treatment of chronic sinusitis in the frontal and maxillary sinsuses. The Menlo Park, Calif.-based company’s portfolio of steroid-releasing implants are used in patients undergoing ethmoid, frontal or maxillary surgeries to treat chronic sinusitis. “The approval of Propel […]
FDA accepts Ocular’s NDA resubmission for Dextenza
Ocular Therapeutix (NSDQ:OCUL) said today that the FDA accepted the company’s resubmitted New Drug Application for its post-surgical ocular pain reliever, Dextenza. The hydrogel plug, inserted into a patient’s tear duct, is designed to deliver a sustained dose of dexamethasone over 4 weeks following opthalmic surgery. The Bedford, Mass.-based company has had trouble gaining regulatory approval […]
Titan Pharmaceutical’s match-sized implant fights back against opioid addiction
The ongoing opioid crisis in the U.S. is harrowing – 91 Americans die every day from an opioid overdose, according to the Centers for Disease Control & Prevention. Companies are searching for innovative ways to deliver opioid maintenance treatment to patients that are hoping to get clean. Buprenorphine is a compound used for medication-assisted treatment, traditionally administered […]
Chinese FDA updates recall process for medical devices
By Stewart Eisenhart, Emergo Group Medical device regulators at the China Food and Drug Administration (CFDA) have issued a new provision on its recall process for defective devices. Get the full story here at the Emergo Group’s blog. The opinions expressed in this blog post are the author’s only and do not necessarily reflect those […]