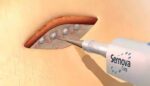
A team of researchers developed an implantable fuel cell that uses excess glucose from tissue to generate electrical energy. By combining the fuel cell with artificial data cells, the team produced insulin with the touch of a button. This effectively lowered blood glucose levels like the natural process in the pancreas. Given that current treatments […]
Implants
This implantable drug delivery system is self-powered
Researchers at Shirley Ryan AbilityLab and Northwestern University developed technology that uses external light sources to trigger drug delivery. Yamin Zhang and Dr. Colin Franz of Shirley Ryan and John Rogers of Northwestern led the research team. They say their technology represents the first implantable drug delivery system triggered by external light sources. These light […]
Vivani Medical wants to bring a subdermal drug delivery implant to the diabetes market
Where some have tried and failed, Vivani Medical believes its drug-delivering implants can go the distance. When Second Sight Medical and Nano Precision Medical merged last year, the rebranded company — Vivani Medical (Nasdaq:VANI)— had a goal: bring a six-month, subdermal drug delivery implant that treats conditions including type 2 diabetes to market. CEO Dr. […]
Glaukos submits FDA New Drug Application for iDose TR drug-eluting implant
Glaukos (NYSE:GKOS) announced today that it submitted a New Drug Application (NDA) to the FDA for its iDose TR implant. Aliso Viejo, California-based Glaukos designed iDose TR as a micro-invasive intraocular implant. It continuously delivers therapeutic levels of a proprietary formulation of travoprost from within the eye over extended periods of time. Once all travoprost […]
Evonik invests in Allay Therapeutics to reduce need for opioids
Evonik announced today that it invested in Allay Therapeutics and its implant for treating pain after knee surgery. Inserted directly into the knee, the Allay implant may relieve pain for up to three weeks, according to a news release. Previous solutions worked for a maximum of three days, the company said. The technology helps promote […]
Cell Pouch System developer Sernova announces CEO transition
Sernova announced today that, as part of a planned leadership succession process, Dr. Philip Toleikis will transition out of the CEO role. Toleikis spent 13 years leading the Cell Pouch System developer as president and CEO. He remains an executive at the company as the planned transition moves him to chief technology officer (CTO). Additionally, […]
How Sernova seeks to treat insulin-dependent diabetes with its Cell Pouch System
When Dr. Philip Toleikis joined Sernova in 2009, the company’s technology appeared as more of a concept than anything else. But, he saw the potential for something groundbreaking: A cure for type 1 diabetes (T1D). “I just thought it was an incredible idea,” said Toleikis, who serves as Sernova’s president and CEO. “We spent a […]
Glaukos beats The Street in Q3
Glaukos (NYSE:GKOS) shares hardly shifted on third-quarter results that came in ahead of the consensus forecast. The Aliso Viejo, California-based ophthalmic implant maker posted losses of $27.6 million in the quarter. That amounts to 58¢ per share on sales of $71.3 million for the three months ended Sept. 30, 2022. Glaukos registered a deep bottom-line […]
BioCorRx completes enrollment in trial of naltrexone implant for treating opioid use disorder
BioCorRx (OTC:BICX) announced today that it enrolled the last subject in the Phase I clinical trial of its BICX104 implant. Anaheim, California-based BioCorRx designed BICX104 for the treatment of opioud use disorder (OUD). Its BioCorRx Pharmaceuticals company developed the implantable, biodegradable naltrexone pellet. “We are pleased to have reached this significant milestone,” said Brady Granier, […]
Rescue Biomedical wins $2.8M grant for nalaxone-releasing implant for opioid overdoses
Rescue Biomedical announced today that it received a $2.8 million grant from the National Institutes of Health for its opioid overdose treatment. West Lafayette, Indiana received a Fast-Track Small Business Innovation Research, or SBIR, grant from the NIH. The company develops technology that detects when a person is overdosing on an opioid and delivers naloxone […]