Impel NeuroPharma Inc. said today that it closed a $36 million series C financing round to fund its pipeline of precision olfactory delivery (POD) intranasal devices. Life science firms venBIO, 5AM Ventures and Vivo Capital made investments in the round. Seattle-based Impel will get $21 million upfront and $15 million after it reaches development and […]
Pharmaceuticals
Modification to delivery polymer sidesteps allergic response
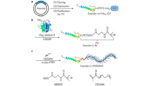
Researchers from Duke University have reconfigured the popular drug-delivery polymer, polyethylene glycol, to sidestep dangerous immune responses that have previously halted clinical trials. The team’s work was published in Nature Biomedical Engineering. Polyethylene glycol, PEG, is a polymer that is found in toothpaste and cosmetics, but is often used in pharmaceuticals. It can be attached to […]
Actelion rejects J&J’s takeover offer
Swiss biotech firm Actelion Pharmaceuticals (VTX:ATLN) reportedly rejected Johnson & Johnson‘s (NYSE:JNJ) 2nd takeover offer, sending it shares down -6% in afternoon trading yesterday. The 2 companies have been engaged in informal talks for 2 months, according to Reuters, and the latest offer was pegged at 250 Swiss francs per share. J&J’s $270 billion bid for Actelion tempted shareholders, […]
BD’s Pyxis ES drug pump adopts Internet of Things
Becton Dickinson (NYSE:BDX) said today that it adopted the Windows 10 Internet of Things operating system for its automated drug-dispensing Pyxis ES system. The new operating system features long-term support and improved cybersecurity. The Windows 10 Internet of Things operating system boasts a 10-year support package and active security patches. It also offers new features […]
Researchers develop method for storing vaccines at room temp
Researchers at Ecole Polytechnique Federale de Lausanne‘s Supramolecular Nanomaterials and Interfaces Laboratory claim they have developed methods for 3 vaccine additives to help store vaccines at room temperature. The team’s work was published in Nature Communications. The need to keep vaccines within a temperature range of 2-8°C contributes to low immunization-coverage rates, because shipping vaccines in […]
Report: J&J raises the stakes in Actelion pursuit
Johnson & Johnson (NYSE:JNJ) has reportedly raised its offer for Swiss biotech firm Actelion Pharmaceuticals (VTX:ATLN), pressuring the company to accept a takeover deal. The companies have been in talks for nearly 2 months but so far have not reached a deal, according to Reuters. The news outlet reported that Actelion wants J&J to become a major shareholder […]
Nordisk touts Tresiba insulin injection data
Novo Nordisk (NYSE:NVO) said today that data from the Devote clinical trial indicated that its injected insulin, Tresiba, reduced the risk of severe hypoglycemia when compared to insulin glargine. The trial enrolled more than 7,500 type 2 diabetes patients at high risk of adverse cardiovascular events and treated them for 2 years with either Tresiba or insulin glargine […]
Counterfeit prescription drug tracker raises $52m
TraceLink Inc. said yesterday that it raised $51.5 million in a Series C financing round, bringing its total raised to $77 million. The round was led by Goldman Sachs Growth Equity, joining FirstMark Capital, Volition Capital and F-Prime Capital. TraceLink developed a cloud-based global network of manufacturers, distributors, pharmacies and healthcare professionals. The firm’s system enables […]
Smart patch monitors blood, releases blood thinners to prevent clots
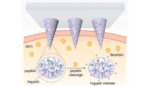
Researchers from the North Carolina State University and the University of North Carolina at Chapel Hill have developed a patch designed to monitor a patient’s blood and release blood-thinning drugs to prevent dangerous blood clots, or thrombosis. The team’s work was published in Advanced Materials. When blood clots disrupt the flow of blood throughout the body, […]
Oramed touts phase Ib data for type 2 diabetes capsule
Oramed Pharmaceuticals (NSDQ:ORMP) said today that it finished a phase Ib study of its oral GLP-1 analog capsule for type 2 diabetes. Glucagon-like peptide-1 (GLP-1) is a hormone that stimulates the pancreas to secrete insulin. Exenatide, a GLP-1 analog, is available on the market as an injection for patients with type 2 diabetes. The analog […]