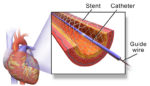
Sinomed has completed the first commercial implantation of its HT Supreme drug-eluting stent in Ireland. Faisal Sharif, professor of translational cardiovascular medicine and innovation at the National University of Ireland Galway, successfully performed the first procedure with HT Supreme, a healing-targeted drug-eluting stent designed to treat patients with narrowing or blockages to their coronary arteries. […]
Stents
Boston Scientific initiates coronary drug-coated balloon study in U.S.
Boston Scientific (NYSE:BSX) this week launched its Agent IDE trial for its Agent drug-coated balloon. The U.S. prospective, randomized clinical trial will evaluate the safety and effectiveness of a drug-coated balloon (DCB) in patients with coronary in-stent restenosis in lesions up to 26 mm in length in a coronary artery 2.0 mm to 4.0 mm in […]
Boston Scientific to expand its Maple Grove, Minn. campus
Boston Scientific (NYSE:BSX) is planning a fourth building at its Maple, Grove, Minn. campus to support expanded nitinol stent manufacturing. The Maple Grove Planning Commission is scheduled to review project plans tonight for the roughly 76,000 ft2 building. “This new building will allow for movement of some production currently taking place in existing buildings on that campus, […]
Abbott touts Xience DES study results
A pair of studies of Abbott’s Xience drug-eluting stent showed no difference between shorter courses of treatment with dual antiplatelet therapy (DAPT) compared with 12 months of DAPT following implantation of the XIENCE drug-eluting stent (DES) in patients who are at a high risk of bleeding. Abbott presented the late-breaking data today at TCT Connect, the […]
Medinol stent performs just as well as Medtronic Resolute, study says
The EluNIR drug-eluting stent made by Medinol performed just as well as the Resolute Integrity or Resolute Onyx stents from Medtronic (NYSE:MDT), according to new clinical study results from an international team of researchers. The randomized clinical trial — conducted by researchers from the U.S., Israel, Canada and the Netherlands — involved 2,221 people and compared […]
China approves Biotronik’s Orsiro drug-eluting stent
Biotronik said today that China’s National Medical Products Administration approved its Orsiro drug-eluting stent. Orsiro is a cobalt-chromium stent that elutes the drug sirolimus via the Berlin-based company’s Biolute bioabsorbable polymer coating. Biotronik said it plans to have Orsiro on the Chinese market “in the coming months.” “Orsiro will be a valuable addition to our […]
TCT 2019 Roundup: Medtronic’s Resolute Onyx non-inferior to Biosensors BioFreedom stent
A study comparing the Resolute Onyx drug-eluting stent from Medtronic (NYSE:MDT) and the BioFreedom stent made by Biosensors International (PINK:BSNRY) showed the Medtronic device to be non-inferior in patients on a one-month dual anti-platelet therapy regimen, according to results presented yesterday at the annual Transcatheter Cardiovascular Technologies conference in San Francisco. Medtronic launched the 2,000-patient study […]
Japan OKs OrbusNeich’s Combo Plus stent
OrbusNeich Medical today said it won market approval in Japan for its Combo Plus coronary stent. The Combo Plus stent is designed to address the risk of stent thrombosis associated with delayed healing in conventional drug-eluting stents. The Combo Plus DES uses endothelial progenitor cell capture technology and abluminal sirolimus drug elution delivered from a […]
Cook Medical touts paclitaxel-coated stent PAD study results
Cook Medical today touted five-year data from a study into the use of its Zilver PTX paclitaxel-coated stent for peripheral arterial disease (PAD). The study concluded that there was no increase in long-term all-cause mortality due to paclitaxel after treatment using the Zilver PTX stent compared to traditional angioplasty or a bare-metal stent, according to a […]
Study: No difference between Biotronik, Medtronic or Boston Scientific stents
A study comparing drug-eluting stents made by Biotronik, Medtronic (NYSE:MDT) and Boston Scientific (NYSE:BSX) found no statistically significant difference on target vessel failure or stent thrombosis after three years. The 3,514-patient Bio-Resort trial compared Biotronik’s Orsiro biodegradable sirolimus-eluting stent, Medtronic’s thin-strut zotarolimus-eluting Resolute Integrity stent and the Synergy biodegradable everolimus-eluting stent. Three-year results for some 3,393 […]