MIT-spinoff Organogenesis announced today that its PuraPly antimicrobial wound care device is now available in a 1.6-cm disc size. The Canton, Mass.-based company’s PuraPly AM product includes a broad-spectrum antibiotic, polyhexamethylene biguanide, embedded into a purified native collagen matrix. The device is cleared to treat an array of wound types, including pressure ulcers, surgical wounds, trauma wounds […]
Wound Care
Ex-Advanced BioHealing exec draws probation in VA fraud case
A former Advanced BioHealing executive who admitted to federal charges that he bribed U.S. Veterans Affairs Dept. doctors to use the company’s DermaGraft biologic wound dressing last week drew probation in the case. Federal had prosecutors accused Todd Clawson, who left the company in 2012, of participating in a scheme to bribe VA physicians to use the DermaGraft diabetic […]
Pertinax Pharma tackles wound care, dental health and more with its controlled-release antiseptic
For nearly a decade, Michele Barbour and her team of researchers at the University of Bristol have been developing controlled-release salts of a commonly-used chemical called chlorhexidine. Barbour believed her technology had enormous potential – so she decided to start a company. “When you’re an academic, you have a lot of freedom but you also […]
MediWound raises $25m
MediWound (NSDQ:MDWD) today announced a $25.2 million offering to support research and development of its EscharEx topical biological drug for debridement of chronic and other hard-to-heal wounds. In the offering, the Israel-based company floated approximately 5 million shares, including 637,664 shares through an underwriter’s option, according to an SEC filing. Cowen & Company and Wells Fargo […]
Meet the two former Johns Hopkins residents disrupting the wound care market
Ned Swanson and Denver Lough were plastic surgery residents at Johns Hopkins when they made a choice that would change the course of their lives – they decided to drop out and start a business. Lough had a technology that they thought could help patients if it ever made it to the market, but their […]
The 11 most innovative medical devices of 2017
The nominees for the best medical technology of 2017 were recently announced for the 11th Annual Prix Galien USA Awards. The Galien Foundation, the host of the awards, hands out the Prix Galien Award annually to examples of outstanding biomedical and technology product achievement designed to improve human condition. Before candidates can qualify for the […]
Avita Medical aims for $13m to bring ReCell burn injury device to U.S.
Avita Medical (ASX:AVH) said today that it hopes to raise $13.2 million to fund its efforts to bring its ReCell autologous cell harvesting device for treatment of burn injuries to the U.S. The Australia-based company said it received commitments from investors for a $3.5 million private placement. Avita added that it plans to undertake a rights […]
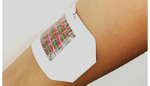
Smart bandage releases medication as needed
A team of researchers from the University of Nebraska, Harvard Medical School and MIT have created a smart bandage that can be loaded with antibiotics, painkillers or other drugs and triggered by a smartphone. The bandage, made of electrically conductive fibers individually coated in a drug-loaded hydrogel, could be used to deliver multiple drugs at […]
FDA expands protocol for investigational ReCell burn injury device
Avita Medical (ASX:AVH) said today that the FDA approved a supplement to the company’s investigational device exemption for its ReCell autologous cell harvesting device. The approved treatment protocol for burn injuries has been simplified, according to the company, and the number of approved investigational sites has grown from eight to 15. Up until now, treatment using […]
Avita Medical submits PMA app for ReCell burn injury treatment
Avita Medical (ASX:AVH) said today that it submitted its pre-market approval application to the FDA for its ReCell autologous cell harvesting device. The company’s system is designed to reduce the amount of skin harvesting needed to treat burn injuries compared to conventional treatments. The PMA application is supported by clinical data from two trials. The […]