 iSTAR Medical announced today that the FDA granted investigational device exemption to start a pivotal trial with its MINIject implant.
iSTAR Medical announced today that the FDA granted investigational device exemption to start a pivotal trial with its MINIject implant.
Wavre, Belgium–based iStar’s MINIject minimally invasive implant for glaucoma surgery will be investigated in the STAR-V study, enrolling over 350 patients with primary open-angle glaucoma.
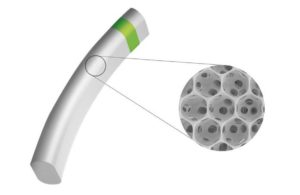
MINIject combines the porous structure of iSTAR’s proprietary STAR material with the power offered by the supraciliary space to enhance natural fluid outflow, reduce intraocular pressure and the need for medication while bio-integrating with surrounding tissue, limiting inflammation, fibrosis and subsequent complications.
MINIject is iSTAR Medical’s revolutionary MIGS device for patients with primary open-angle glaucoma. MINIject combines the unique porous structure of its proprietary STAR material with the power offered by the supraciliary space. As a result, it is designed to enhance natural fluid outflow, reducing intraocular pressure (IOP) and the need for medication while bio-integrating with surrounding tissue, limiting inflammation, fibrosis and subsequent complications.
According to a news release, STAR-V will evaluate MINIject’s efficacy by the mean reduction in eye pressure and by the proportion of patients achieving at least a 20% reduction in eye pressure.
iStar said the study will report on the safety and efficacy of MINIject alone in a procedure not combined with simultaneous cataract surgery. Key study findings will become available when all patients complete two years in the study, with follow-up to evaluate long-term benefits and tolerability with MINIject in the treatment of mild to moderate glaucoma.
“We are very pleased that the FDA has granted us approval to bring this innovative technology to North American patients suffering from primary open-angle glaucoma in the STAR-V trial,” iSTAR Medical CEO Michel Vanbrabant said in the release. “Results from clinical trials in over 130 patients in Europe, Asia and Latin America have consistently demonstrated that MINIject maintains a positive safety profile and delivers a significant reduction of pressure thanks to our proprietary STAR material and the power of the supraciliary space.”

