 Meril Life Sciences has developed a drug-eluting, bioresorbable vascular scaffold that the body can slowly degrade and wash away.
Meril Life Sciences has developed a drug-eluting, bioresorbable vascular scaffold that the body can slowly degrade and wash away.
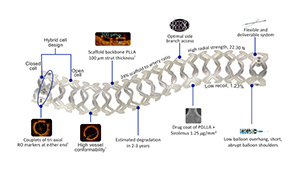
The company’s MeRes100 is made of biodegradable polymers, PLLA and PDLLA. Sirolimus, a commonly-used immunosuppressant found in drug eluting stents, is embedded in the scaffold’s coating.
The company designed the struts of the device to be just 100 μm thick, while still providing the strength needed to maintain an open lumen.
In October last year, Meril touted 6-month data from the 1st-in-man study of the MeRes100 scaffold in patients with coronary artery disease at the annual Transcatheter Cardiovascular Therapeutics conference in Washington, D.C.
The data showed that at 6 months, the scaffold was safe and effective with no major adverse cardiac events.
The trial enrolled 108 patients at 16 sites across India from May 2015 to April 2016. Researchers reported no major adverse cardiac events up to the 6-month follow-up. Quantitative coronary analysis data showed favorable in-scaffold late lumen loss, according to Meril.
“The innovative design of the MeRes100 scaffold developed in India addresses some of the limitations of currently available [bioresorbable scaffolds],” chairman of the Fortis Escorts Heart Institute Ashok Seth said in prepared remarks. “The MeRes-1 first-in-man study demonstrates that this new generation thinner-strut sirolimus-eluting BRS is safe and effective at 6 months. These encouraging results provide the basis for further studies using a wider range of length and sizes in more complex and larger patient population moving to a randomized pivotal trial against the second-generation metallic drug-eluting stents by mid 2017.”

