Los Altos, California-based RenovoRx said Gentry brings with her “valuable leadership experience” to advance the company’s cancer treatment studies. She becomes the fourth addition to the company’s executive management team in the last year. That group includes COO Angela Gill Nelms, CFO James Ahlers and VP and controller Ron Kocak.
Across 29 years of experience in clinical research programs, Gentry spent time in the CRO, pharmaceutical and biotech industries. Past employers include IQVIA (Quintiles), PPD, OmnicareCR and Otsuka. She served as SVP of clinical operations at Evotec. At PPD, Gentry served as global project manager and senior research specialist for clinical systems.
While at Omnicare, Gentry grew the company’s clinical research site network with long-term care, RenovoRx said. At Otsuka, she developed novel methods for improved, streamlined trial implementation in clinical operations, data management and third-party collaboration.
More on new RenovoRx SVP of clinical operations Leesa Gentry
RenovoRx CEO Shaun Bagai called Gentry “an outstanding clinical operations leader.” He added that her “decades of diverse experience” can make an immediate impact on the company’s clinical trials.
“As we continue to build momentum on the heels of our positive, Phase III interim analysis data in pancreatic cancer treatment, we will look to her guidance to enhance our current and future trials,” Bagai said. “We are confident in Leesa’s track record for successful regulatory submissions, late-stage trial preparation, and clinical execution. She will be invaluable in our quest to increase patient lifespans and improve quality of life by developing innovative oncology therapies.”
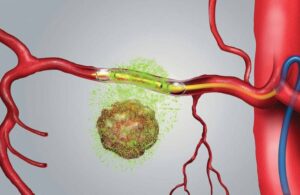
RenovoRx develops the RenovoGem drug-device combination for treating pancreatic cancer. Recent interim data from the Phase III open-label study suggests a six-month potential improvement in median overall survival with RenovoGem.
RenovoGem utilizes the RenovoRx proprietary RenovoTAMP therapy platform. It provides targeted intra-arterial delivery of FDA-approved chemotherapy, known as gemcitabine. This treats LAPC following stereotactic body radiation therapy (SBRT). The pressure-mediated delivery technology delivers the therapy locally, across the arterial wall to bathe tumor tissue in chemotherapy.
“RenovoRx is changing the current standard of care for locally advanced pancreatic cancer (LAPC), and I am excited to be a part of the team’s mission,” said Gentry. “With interim data demonstrating clinically significant improvement in overall survival and quality of life for patients undergoing RenovoGem treatment, the team’s progress is impressive. Based on the Company’s pipeline and expected clinical milestones, RenovoRx has tremendous potential for helping patients with difficult-to-treat cancers.”

