 Ethosomes – nanocarriers loaded with ethanol – can penetrate the skin’s protective layers and deliver a variety of drugs, a review published in Current Clinical Pharmacology reported today.
Ethosomes – nanocarriers loaded with ethanol – can penetrate the skin’s protective layers and deliver a variety of drugs, a review published in Current Clinical Pharmacology reported today.
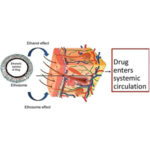
Transdermal delivery of drugs is touted by some researchers as promising, since it’s noninvasive and avoids any interaction with the gastrointestinal tract. Ethosomes are malleable and their ethanol-filled core allows them to disrupt the skin’s protective lipid bilayer.
The review highlighted preclinical and clinical trials that demonstrate the nanovesicles’ efficacy, including delivering antimicrobials and anti-inflammatory drugs with enhanced penetration and diffusion. The review’s authors also point to drugs that act on the central nervous system – when delivered using an ethosome, the drugs exhibited enhanced absorption and bioavailability.
Some researchers have questioned the safety of ethosomes, the authors wrote, but they said they found no adverse skin reactions in any of the evaluated clinical trials. They also report that post-marketing data has not revealed any other adverse reactions.
Several permeability analyses with microscopy tools showed that the ethosomes can penetrate and efficiently diffuse through the skin carrying hydrophilic and lipophilic drugs. The authors wrote that the versatility of the nanocarriers, their malleability and ability to penetrate the skin effectively, makes them a promising transdermal drug delivery vehicle.
“From various research reports, it can be concluded that ethosomes are the competent and efficient vesicles to provide better drug transport via transdermal route,” they wrote.

