Abbott (NYSE:ABT) today broke ground on a new FreeStyle Libre manufacturing facility in Kilkenny, Ireland. Jared Watkin, SVP for Abbott’s diabetes care business, shared the news of the groundbreaking in a LinkedIn post. In August 2022, Ireland announced Abbott’s plans to spend €440 million ($450 million) to expand its operations in Ireland. The investment included […]
abbott
FDA clears reader for next-gen Abbott FreeStyle Libre 3
Abbott (NYSE:ABT) announced today that the FDA cleared a reader device for its FreeStyle Libre 3 continuous glucose monitoring (CGM) system. The FDA cleared Abbott’s next-generation FreeStyle Libre 3 in May 2022. Abbott designed FreeStyle Libre 3 as the smallest and thinnest CGM sensor in the world. The system constitutes the size of two stacked […]
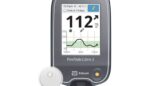
Abbott FreeStyle Libre reader warning affects more than 4 million devices
The FDA today issued a notice classifying a recall of Abbott (NYSE:ABT) FreeStyle Libre readers as Class I, the most serious kind. Earlier this week, Abbott initiated a voluntary medical device correction to emphasize instructions for FreeStyle Libre continuous glucose monitor (CGM) readers. The company said it received a limited number of global reports (0.0017%) from […]
Abbott warns on some FreeStyle Libre readers due to battery issues
Abbott (NYSE:ABT) initiated a voluntary medical device correction to emphasize instructions for FreeStyle Libre continuous glucose monitor (CGM) readers. The instructions cover the FreeStyle Libre, Libre 14-day and Libre 2 readers in the U.S. Abbott received a limited number of global reports (0.0017%) from users over several years saying their reader’s lithium-ion battery swelled or […]
FDA clears Abbott FreeStyle Libre for automated insulin pump integration
Abbott (NYSE:ABT) announced today that the FDA cleared its FreeStyle Libre 2 and FreeStyle Libre 3 for automated insulin delivery (AID) integration. AID systems help people manage daily diabetes care by automatically adjusting and administering the insulin delivered by an insulin pump based on real-time glucose data from their FreeStyle Libre 2 or FreeStyle Libre […]
Expanded Medicare coverage for CGM to go into effect next month
The Centers for Medicare & Medicaid Services today published a final local coverage determination (LCD) expanding coverage for CGMs. New, expanded coverage of continuous glucose monitors (CGMs) under Medicare go into effect in April. CMS first published the LCD modifying coverage criteria for CGMs in October. The modification includes people with diabetes who receive insulin […]
Report: Apple moves forward with glucose monitoring tech
Apple reportedly believes it could shake up the CGM market with non-invasive glucose monitoring through the Apple Watch. Blooomberg reported that the company has a “moonshot-style” project in the works, according to “sources familiar with the matter.” The report said the tech giant still has years ahead in this development of the technology, which wouldn’t […]
Abbott is bullish on FreeStyle Libre growth in 2023
Abbott (NYSE:ABT) plans to “go more aggressively” into the continuous glucose monitor (CGM) market with its FreeStyle Libre 3 this year. Chair and CEO Robert Ford said on the company’s most recent earnings call that the company expects growth for the platform this year. FreeStyle Libre 3 received FDA clearance in May 2022. Ford cited […]
The year ahead in diabetes care: what to expect in 2023
After a banner year for diabetes technology in 2022, there’s still plenty to look forward to in 2023. Throughout 2022, we saw a wide range of advancements in diabetes technology. We saw launches for next-generation technologies, exciting clinical trial results, rumored spinouts and acquisitions and more. You can read all about the biggest diabetes stories […]
The 10 biggest diabetes tech stories from 2022
2022 represented a landmark year for next-generation diabetes tech. Here are the biggest stories from the past year. Some companies picked up major regulatory nods. Others experienced major regulatory setbacks. Partnerships were formed and ended, while mergers and acquisitions came and went. There were many stories to choose from in the world of diabetes tech, […]