Genentech today touted two-year data evaluating the use of its Evrysdi therapeutic in infants with symptomatic Type 1 spinal muscular atrophy (SMA). South San Francisco, Calif.-based Genentech’s Firefish Phase 2/3 global study for Evrysdi (risdiplam) in infants between 1-7 months old showed that the orally administered (liquid or feeding tube) therapeutic continued to improve motor […]
Roche
BioCorp, Roche launch Mallya smart insulin pen in France
BioCorp and Roche Diabetes Care France announced the launch of the Mallya smart insulin pen device in France. Developed by BioCorp, Mallya is an intelligent sensor for insulin injection pens that has CE mark approval in Europe. The platform is compatible with any disposable insulin pen. It enables reliable monitoring for doses selected for injection and it offers […]
Roche, Diabeloop partner on insulin pump management
Roche (SWX: RO, ROG) announced today that it is partnering with Diabeloop to advance insulin pump therapy management. Basel, Switzerland-based Roche said in a news release that the partnership will create new opportunities to lower the burden of constant insulin dose adjustment for those with diabetes while also improving therapy outcomes. Roche also said the partnership with Paris-based […]
Senseonics extends distribution agreement with Roche Diabetes Care
Senseonics Holdings (NYSE-American: SENS) — maker of an implantable continuous glucose monitoring system for people with diabetes — has extended its distribution agreement with Roche Diabetes Care. The agreement extension, announced yesterday, has Roche continuing as Senseonics’ exclusive distributor in Europe, the Middle East and Africa — excluding Scandinavia and Israel. The agreement also adds 17 […]
Genentech touts data for refillable eye implant in wet-AMD trial
Roche‘s (OTC:RHHBY) Genentech touted additional positive data last week from a Phase II study of its Port Delivery System, a refillable eye implant designed to deliver ranibizumab to treat wet age-related macular degeneration. The company first reported top-line results from the Phase II Ladder study in July. The implant is intended to allow wet-AMD patients to go […]
Genentech kicks off Phase III trial for eye implant
Roche‘s (OTC:RHHBY) Genentech reported this week that the company launched Phase III trials evaluating its Port drug-delivery implant in patients with wet age-related macular degeneration and the drug faricimab in patients with diabetic macular edema. The company’s Port device is a refillable eye implant designed to continuously release drugs over a period of several months. “Wet […]
Genentech touts positive data for tiny, refillable eye implant
Roche‘s (OTC:RHHBY) Genentech touted positive top-line data today from a Phase II study of its Port Delivery System, an eye implant designed to administer a sustained dose of ranibizumab in patients with wet age-related macular degeneration. The refillable device, which is slightly longer than a grain of rice, is intended to enable wet-AMD patients to go for […]
Roche wins CE Mark for Accu-Chek Solo insulin micropump
Roche (OTC:RHHBY) said today that it won CE Mark clearance for its Accu-Chek Solo insulin micropump system. The company plans to launch a pilot commercialization phase for its device in the coming weeks in Austria, Poland, Switzerland and the U.K. Roche’s Accu-Chek Solo system features a small, tubeless insulin micropump and a remote control that incorporates […]
Companies push back against allegations of FCA violations over excess at-home blood testing
A 70-year-old former Marine is taking a group of companies to court over their at-home blood testing services, arguing that the industry players broke the law by requiring patients to conduct weekly tests. The companies then submit these bills to Medicare for reimbursement, even asking to be repaid for services that weren’t rendered, according to […]
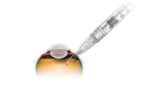
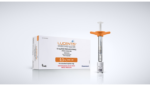
Genentech’s pre-filled syringe for diabetic retinopathy wins FDA nod
Roche‘s (OTC:RHHBY) Genentech announced today that the FDA approved its 0.3-mg pre-filled syringe as a new way to administer Lucentis to diabetic retinopathy patients. The company’s drug is the only FDA-approved medicine indicated for the treatment of diabetic retinopathy. The 0.3-mg pre-filled syringe is the first device of its kind approved to deliver an anti-vascular endothelial […]