 Researchers from the Wyss Institute developed a hydrogel microenvironment to evaluate how physical properties like stiffness of the extracellular matrix impacts the efficacy of chemotherapy.
Researchers from the Wyss Institute developed a hydrogel microenvironment to evaluate how physical properties like stiffness of the extracellular matrix impacts the efficacy of chemotherapy.
“To have success with chemotherapy and other drug therapies, we will likely need to screen their effectiveness against cells living in various environments, and not just assume that cells will always respond to a drug the same way that they would in conventional cell culture,” senior author David Mooney said in prepared remarks.
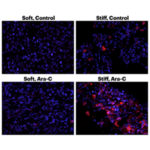
The study, published yesterday in Proceedings of the National Academy of Sciences, describes a drug screen assay using alginate hydrogels that were tuned to recapitulate stiffness and other properties characteristic of tumors and healthy tissue. Cancer cells were loaded into the hydrogel and bombarded with chemotherapy, according to the study, and researchers evaluated the drug’s efficacy based on the level of resistance the cells build up.
They found that even when they tested the hydrogel in mice, a softer extracellular matrix accelerated cancer growth and led to more cellular resistance to the chemotherapy.
“We envision that our 3D hydrogels could bridge the gap between in vitro drug screening and in vivo pre-clinical studies,” Jae-Won Shin, assistant professor at the University of Illinois at Chicago, added.
“It is crucial that we consider the importance of the local tissue microenvironment when designing new cancer therapies, as we now understand that mechanics is as important as biochemistry,” founding director Dr. Donald Ingber said. “These engineered 3D hydrogels, which enable investigators to mimic the mechanical environment of cancers in different organs, also potentially offer a new approach to developing precision medicine therapies being that could be tailored to patient-specific tumor environments.”

