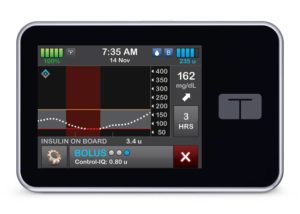
Tandem Diabetes Care (Nasdaq:TNDM) announced today that it released version 2.8.2 of its Apple iOS t:connect mobile app in the U.S.
The company initiated the new software release to remedy issues with prior versions of the technology. Its t:connect mobile app works in conjunction with the t:slim X2 automated insulin pump with Control-IQ technology. Tandem encourages all impacted iOS users to update their mobile app as soon as the app becomes available in the Apple App Store.
In March, the San Diego-based company initiated a recall of Version 2.7 of the Apple iOS t:connect mobile app. Tandem recalled the software version because of an issue that may cause the mobile app to crash and be automatically relaunched by the iOS operating system.
This cycle intermittently repeats, which leads to excessive Bluetooth communication, according to Tandem. That may result in pump battery drain, potentially leading to the pump shutting down sooner than typically expected. Pump shutdown could cause insulin delivery to suspend, which may lead to an under-delivery of insulin. That, in turn, could result in hyperglycemia or diabetic ketoacidosis, a potentially life-threatening condition due to high blood sugars and lack of insulin.
Recently, Tandem communicated that users of the initial update to Version 2.7 (V2.7.1) continued to experience these issues. The company issued updated information to impacted customers on Aug. 9 and provided recommendations for avoiding battery depletion.
In total, Tandem reports 143 confirmed adverse events related to the recall, including two hospitalizations and zero deaths.
