Eli Lilly (NYSE:LLY) announced today that it agreed to acquire a manufacturing facility from sterile manufacturer Nexus. Buying the FDA-approved facility in Pleasant Prairie, Wisconsin, further expands Lilly’s global parenteral (injectable) manufacturing network. It also supports increased demand for the company’s medicines, Lilly said in a news release. The company expects that production at the […]
Pharmaceuticals
Ypsomed, ten23 health collab on YpsoDose patch injector
Ypsomed announced today that it entered into a partnership with ten23 health to commercialize its YpsoDose wearable injector. Burgdorf, Switzerland-based Ypsomed designed YpsoDose for the subcutaneous self-injection of large-volume doses. ten23 health, a Swiss CDMO, offers drug development, filling and device assembly expertise to contribute to the product offering. The strategic collaboration sees both parties […]
Real-world data backs Abbott FreeStyle Libre CGM in tandem with GLP-1s
Abbott (NYSE:ABT) today announced data from two real-world studies highlighting the success of its CGM when used with GLP-1 drugs. Data showed that people with type 2 diabetes using GLP-1s with FreeStyle Libre had a greater improvement in HbA1c compared to GLP-1s alone. Abbott presented the findings at the Advanced Technologies & Treatments for Diabetes […]
Vivani has positive data for long-term drug-eluting weight loss implant as it shifts strategy
Vivani Medical (Nasdaq:VANI) today announced positive pre-clinical data on weight loss effects for its long-term exenatide implant. Alameda, California-based Vivani has its miniature, twice-yearly exenatide implant — NPM-115 — under development to treat chronic weight management. The company also disclosed that it included semaglutide as the active pharmaceutical ingredient in its NPM-139 subdermal GLP-1 implant […]
Narcan nasal spray maker Emergent BioSolutions has a new CEO
Emergent BioSolutions (NSYE:EBS) announced today that it appointed Joseph C. Papa as its new president and CEO. Papa’s appointment to the corner office of the Gaithersburg, Maryland-based company went into effect today. He succeeds Haywood Miller, who stepped down from his role as interim CEO today as well. Emergent BioSolutions is known for its Narcan […]
Carthera enrolls first patient in ultrasound drug delivery trial
Carthera announced today that it enrolled the first patient in the SONOBIRD pivotal trial for its SonoCloud device. Paris, France-based Carthera designed SonoCloud to treat a wide range of brain disorders. The device emits ultrasound to temporarily increase the permeability of blood vessels in the brain, improving therapeutic molecule delivery. After implantation in the skull, […]
Kindeva Drug Delivery acquires nasal spray company Summit Biosciences
Kindeva Drug Delivery announced today that it acquired Summit Biosciences, an intranasal drug-delivery company. Summit, a drug delivery CDMO, brings a track record of innovation in the unit dose nasal spray market. Kindeva believes the acquisition enhances its existing drug delivery capabilities by adding a new platform. This new platform enhances its ability to serve […]
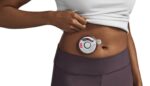
FDA approves wearable injector from Enable Injections
Enable Injections announced today that it received FDA approval for its enFuse injector for the delivery of Empaveli. Empaveli, commercialized by Apellis Pharmaceuticals, treats adults with paroxysmal nocturnal hemoglobinuria (PNH). The injector — in this case, called the Empaveli Injector — offers self-administration of the pharmaceutical. The compact, wearable device streamlines the self-administration experience with […]
Abbott: Real-world data indicates GLP-1s could be accelerator for FreeStyle Libre
New real-world data from Abbott (NYSE:ABT) demonstrates a potentially positive impact of GLP-1 drugs on diabetes technology. GLP-1 receptor agonists, like Ozempic and Wegovy, provide therapy for diabetes and weight loss. This therapeutic class, a glucagon-like peptide 1, has proven to lead to improved blood sugar control and weight loss. The drug class continues to […]
Satio wins $3.5M contract to develop at-home transdermal drug delivery device
Satio announced that a National Institutes for Health agency awarded it a $3.5 million Small Business Innovation Research (SBIR) contract. Awarded by the NIH’s Advanced Research Projects Agency for Health (ARPA-H), the contract helps the company develop SatioRx. This drug delivery device, compact and inexpensive, features disposable microneedle components for precise delivery. It remotely enables […]