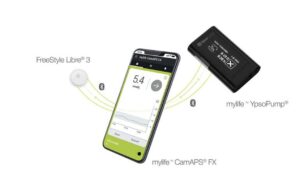
Authorization enables FreeStyle Libre 3 — Abbott’s newest sensor — to work with the Ypsomed mylife YpsoPump and CamDiab’s CamAPS FX mobile app. The company called this a “major step forward” for type 1 diabetes management in the United Kingdom.
At the end of last year, the company received authorization to integrate FreeStyle Libre 3 with the mylife Loop from Ypsomed and CamDiab. That collaboration began in Germany. Abbott said at the time that it planned launches in the UK, Switzerland and the Netherlands in the first half of this year.
Combined, the technologies form an intelligent process to deliver insulin in the form of an artificial pancreas. The closed-loop system utilizes real-time glucose data to create an automated insulin delivery (AID) system. It removes the guesswork of insulin dosing and helps people with type 1 diabetes improve quality of life and reach better treatment targets.
In March, the FDA cleared the FreeStyle Libre 2 and FreeStyle Libre 3 for AID integration. Abbott said that, in addition to this partnership, it’s looking to make FreeStyle Libre interoperable with other leading insulin delivery systems.
More about the Abbott authorization in the UK
This system marks the first hybrid closed-loop system to work with the FreeStyle Libre 3. It incorporates technology available from the NHS in the form of FreeStyle Libre 3, Ypsomed’s insulin pump and CamDiab’s app algorithm.
CamDiab’s app automatically adjusts insulin dosage on the Ypsomed pump based on FreeStyle Libre 3 readings. The pump then delivers the insulin.
The National Institute for Health and Care Excellence (NICE) recommended the use of hybrid closed-loop systems in draft guidance published in January 2023 with final guidance due to be published this summer, according to a news release.
“Our mission has always been to provide affordable and accessible technologies that help as many people as possible. We introduced FreeStyle Libre technology to Europe as the most affordable sensor, with the longest wear time on the market to help reduce some of the mental load that comes with managing the condition,” said Neil Harris, GM of Abbott’s diabetes care business in the UK and Ireland. “This collaboration brings users of FreeStyle Libre 3 sensors in the UK a hybrid closed loop system that will allow them to spend even less time thinking about their diabetes and more time enjoying life.”
More about FreeStyle Libre 3
FreeStyle Libre 3 received FDA clearance in May 2022. The latest iteration of the company’s FreeStyle Libre platform — designed as the smallest and thinnest CGM sensor in the world — covers use by people 4 years of age and older living with diabetes.
The CGM offers a 7.9% mean absolute relative difference (MARD). Abbott said the MARD measure of accuracy makes FreeStyle Libre 3 the most accurate 14-day CGM with readings sent directly to a smartphone every minute.
Abbott Park, Illinois–based Abbott said FreeStyle Libre 3 is the first CGM to demonstrate a sub-8% MARD and, at the size of two stacked U.S. pennies, the smallest and thinnest CGM sensor in the world is worn inconspicuously on the back of the upper arm and is uncomplicated to apply thanks to a one-piece applicator. FreeStyle Libre 3 also has strong Bluetooth integration, with a range of up to 33 feet representing a 50% increase on competitive CGMs.
The company recently picked up a major win in the U.S. with a Medicare expansion of CGM availability. This modification includes people with diabetes who receive insulin treatment or have a history of problematic hypoglycemia. The proposal eliminates the requirement for frequent adjustments of the patient’s insulin treatment regimen. This falls on the basis of glucose measurement testing.
With its latest generation nearing one year since FDA clearance, CEO Robert Ford said earlier this year that the company plans to make a strong commercial push for the platform.
“For Libre 3, we wanted to go more aggressively in some of these markets,” Ford said on Abbott’s fourth-quarter 2022 earnings call. “I expect continued growth in the U.S. in terms of market expansion. … Can we see a path for 20% growth in 2023? Yeah, I can. I think there’s a lot of opportunities for growth.”

