 Abbott (NYSE:ABT) said today that the FDA approved its 14-day FreeStyle Libre flash glucose monitor, making the device the longest lasting self-applied glucose sensor on the market.
Abbott (NYSE:ABT) said today that the FDA approved its 14-day FreeStyle Libre flash glucose monitor, making the device the longest lasting self-applied glucose sensor on the market.
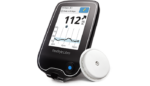
The company’s FreeStyle Libre system, initially approved to last 10 days, is designed to eliminate the need for fingersticks. Users can test their blood sugar with the continuous glucose monitor by simply swiping a phone over a small sensor worn on their upper arm.
“At Abbott, we are continuously pushing for new innovations that minimize the daily burden of managing diabetes,” Jared Watkin, SVP of Abbott’s diabetes unit, said in prepared remarks. “With the new FreeStyle Libre 14 day system, people with diabetes will now have extended access to their glucose data with a high degree of accuracy, which will improve their experience and help empower them to better manage their condition.”
The FreeStyle Libre system was launched in Europe in 2014 and approved in the U.S. just last year. The sensor, which is roughly the size of two stacked quarters, allows users to collect real-time glucose readings and review eight hours of glucose history using a mobile app.
Abbott expects that the latest FreeStyle Libre system will be available via prescription in the U.S. in the coming months. The medtech giant noted that it has won partial or full reimbursement for the system in 30 countries, including France, the UK and the U.S.
