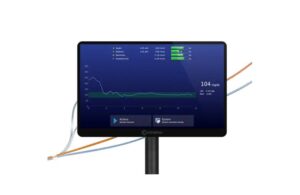
The system — also a continuous blood diagnostics system — aims to fill a key gap in the standard of care. Boston-based Admetsys designed it to automatically measure multiple blood analytes, including glucose, in real time. It requires no patient blood loss.
From the measurement, it creates an adaptive, computational model of each patient’s metabolism. The system evolves with the patient condition, delivering precisely optimized insulin and dextrose treatments through multiple infusion channels.
“The core of the system is its patient-adaptive learning algorithm. Machine intelligence using real-time biosensing data directly drives therapeutic actuation,” said Admetsys CEO Jeff Valk. “This represents a new generation of device: fully autonomous clinical robotics.”
Admetsys says the breakthrough nod accelerates the system’s path to market in the U.S. Valk spoke with sister site MassDevice in 2016 to discuss the company’s goals.
To date, manual workflow limits the ability to provide the insights that the company’s system can offer. Metabolic parameters change rapidly in critical care patients and it’s difficult to capture and model variability accurately and in real-time by hand.
Admetsys said correctly controlling glucose speeds healing at tissue level — a decisive action for recovery in cardiovascular and surgical patients. The company also said it decreases complications and increases survivability.
“The system unlocks a previously unachievable mode of care. It affects this economically, at scale, while preserving focus for clinicians,” said Valk. “Precision automation fundamentally changes the equation.”

