Pulmonx touts data for Zephyr endobronchial valves in emphysema patients
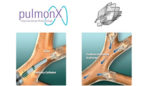
Pulmonx highlighted data from 2 clinical trials of its Zephyr endobronchial valve in emphysema patients without collateral ventilation. Data from the Transform and Impact trials showed clinically meaningful improvements in lung function in patients with either homogeneous or heterogeneous distribution of emphysema.
Zephyr endobronchial valves are minimally-invasive, 1-way valves that are placed in the airways of lungs to occlude diseased regions and reduce lung hyperinflation. The tiny devices help healthier regions function more efficiently, according to Pulmonx.
In both trials, patients were chosen based on the absence of collateral ventilation – airflow between regions of the lung that bypasses normal airways.
The Transform trial enrolled 97 patients with heterogeneous emphysema and randomized them to receive Zephyr valve treatment or standard of care. After 6 months, 56.3% of patients treated with Zephyr valves experienced 12% or greater improvement in lung function from baseline, compared to 3.2% of patients in the control group. Researchers also observed significant improvements in exercise capacity and quality of life in patients treated with Pulmonx’s valves.
“The magnitude of benefits to patients in this pan-European trial are dramatic and reinforce results published from prior single center studies,” principal investigator Dr. Samuel Kemp said in prepared remarks. “Using Chartis for patient selection enabled us to accurately identify patients without collateral ventilation, which is a key predictor for good outcomes with this device.”
The Impact study enrolled 93 patients with severe homogeneous emphysema and randomized them to receive Zephyr valve treatment or medical management. Consistent with previous data, patients experienced improvements in lung function, exercise capacity and quality of life when treated with the company’s devices.
In both studies, the most common side effect of the valves was pneumothorax and COPD exacerbations in the immediate time following the procedure.
“For patients with severe emphysema, there were previously few therapeutic options,” Pulmonx CEO Glen French said. “Now, we have consistent data from 4 randomized studies showing that, regardless of disease heterogeneity, when patients are selected for the absence of collateral ventilation, they can gain substantial benefits in quality of life, exercise capacity and lung function with Zephyr valves.”

