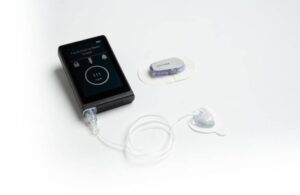
Concord, Massachusetts–based Beta Bionics designed its iLet autonomous insulin delivery system to streamline diabetes management. The system, which reduces the burden on patients and physicians, received FDA clearance in May 2023.
The system uses an adaptive, closed-loop algorithm. The algorithm initializes with the user’s body weight and requires no additional insulin dosing parameters. The algorithm removes the need to manually adjust insulin pump therapy settings and variables.
iLet users only input their weight, then the system does the rest, eliminating the need for healthcare providers to determine correction factors, insulin-to-carb ratios or pre-set basal rates. Users can “go bionic,” requiring no carb counting or insulin correction calculations. Then, iLet determines 100% of the insulin doses throughout the day. The system simplifies mealtime by replacing carb counting with a meal announcement feature. Users can estimate the amount of carbs in their meals as an algorithm learns to respond to individual insulin needs.
Beta Bionics expanded its addressable patient population last year with the U.S. launch of iLet with the Dexcom G7 continuous glucose monitor (CGM). Users can select G7 or the previous-generation G6, or switch back and forth, depending on supplies.
“Reaching 10,000 iLet Bionic Pancreas patients is a testament to our commitment to making diabetes easier, for everyone, every day. By taking the work off their plate, we are empowering patients to live more freely with greater peace of mind. The iLet offers an easier and more reliable way to manage diabetes relative to other insulin delivery devices, and we are thrilled to see its impact grow,” said Sean Saint, Beta Bionics CEO.
