The decision enables faster and easier access to iLet for eligible users with plans that cover the system under their pharmacy benefit. It marks more good news for Beta Bionics, which closed a $100 million funding round a few months ago.
Historically, insulin pumps fall under the durable medical equipment (DME) insurance benefit. This can lead to significant up-front hardware costs to initiate therapy. Coverage under a pharmacy benefit can reduce the potentially large upfront cost of the pump for the patient and payer. The company said this allows patients to upgrade their insulin pump when new technology becomes available.
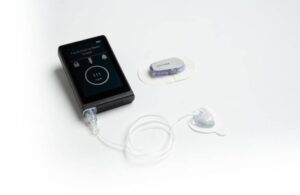
Concord, Massachusetts-based Beta Bionics designed its iLet autonomous insulin delivery system to streamline diabetes management. The system, which reduces the burden on patients and physicians, received FDA clearance in May.
“This is a significant milestone in the growth and rapid commercial launch of the iLet Bionic Pancreas,” said Beta Bionics CEO Sean Saint. “Now that the iLet has been added to the Express Scripts National Commercial Formularies, members of plans that cover the iLet Bionic Pancreas under the pharmacy benefit and select one of these formularies will have a streamlined process as opposed to the traditional DME channel that is standard in the diabetes industry.
“Beta Bionics wants to make it as easy as possible for users to access this transformational device and to open the door for many more people with insulin-dependent type 1 diabetes across the U.S. to gain access to the iLet.”
More about the Beta Bionics iLet system
iLet users only need to input their weight before the system does the rest, eliminating the need for healthcare providers to determine complex settings like correction factors, insulin-to-carb ratios or pre-set basal rates. Users can “go bionic” with their diabetes management, requiring no carb counting or insulin correction calculations. iLet determines 100% of the insulin doses throughout the day.
The system uses an adaptive, closed-loop algorithm that initializes with the user’s body weight and requires no additional insulin dosing parameters. The algorithm removes the need to manually adjust insulin pump therapy settings and variables.
iLet simplifies mealtime use by replacing conventional carb counting with its meal announcement feature. This enables users to estimate the amount of carbs in their meal, categorized as “small,” “medium” or “large.” Over time, the algorithm learns to respond to users’ individual insulin needs.
iLet currently pairs with the Dexcom G6 CGM. It also integrates with an infusion set from Convatec, as announced shortly after FDA clearance. Convatec’s inset, an all-in-one infusion set with a built-in insertion device, connects to iLet on one end and the infusion site at the other. It features a soft, indwelling cannula. Convatec’s contact detach comes with a thin, stainless steel needle. Both infusion sets use kink-free infusion tubing to ensure uninterrupted insulin infusion. They also feature a skin-friendly adhesive tape to secure the set firmly at the infusion site.

