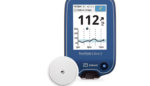
Abbott (NYSE:ABT) announced that Health Canada has approved its FreeStyle Libre 2 continuous glucose monitor for adults and children.
The next-generation, wearable, sensor-based CGM technology with real-time alarms is approved for use in adults and children (four years old and up) and will be priced the same as the current FreeStyle Libre system, according to a news release.
Abbott Park, Ill.-based Abbott touts the alarms as capable of measuring glucose levels every minute over a 14-day wear time, making it long-lasting and eliminating the need for fingersticks.
The sensor for the FreeSytle Libre 2 is worn on the back of the upper arm for 14 days and includes one-second scan capabilities with a handheld reader so users can see glucose readings, trends and eight-hour history.
FreeStyle Libre 2 uses Bluetooth to automatically alert users when glucose is high or low, removing the need to scan the sensor. Users can also turn off the customizable alarms. The system digitally connects and communicates with other compatible devices to allow for diabetes management to be specifically tailored to a person’s needs.
In June, the FDA approved the FreeStyle Libre 2 system for adults and children ages four and older, just months before it was made available to Medicare patients through CMS.
Abbott said the system will be available to Canadians in the coming months.
“For the millions of Canadians with diabetes, Abbott’s next-generation FreeStyle Libre 2 system expands on the life-changing capabilities of our original FreeStyle Libre system with enhanced accuracy, optional alarms and now available for children,” Abbott GM of diabetes care in Canada Marie-Flore Nabor said in the news release. “This latest technology will transform the way diabetes is currently managed. The FreeStyle Libre 2 is designed to simplify this often complicated-to-manage condition and is accessible and affordable to people with diabetes in Canada.”

