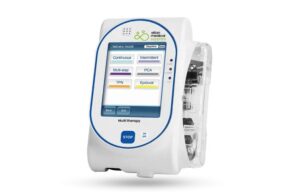
Netanya, Israel-based Eitan recalled certain Sapphire pumps running software version Rev 16.10. The recall includes the Sapphire multi-therapy pump, the Sapphire Epidural pump and the Sapphire Plus pump. Eitan initiated the recall on Sept. 11, 2023, and it affected 1,383 devices in the U.S.
According to an FDA notice, the company recalled the pumps due to software issues with version Rev 16.10. Pumps with this software version may fail to detect air in the line when running on battery power. In some cases, the pump may not sound an alarm when air enters the line. Failure to detect air in the line can lead to a blockage in a blood vessel (embolism). That could lead to unstable blood pressure, stroke, heart attack or even death.
To date, Eitan reports no injuries or deaths associated with this issue.
Eitan instructed customers to cease use of the recalled pumps on battery power. Users should connect the devices to a power supply, like continuous AC power. They should also add an air-eliminating filter to the device.
According to the FDA, the company no longer offers the affected software version. The company intends to provide a new software update correcting the problem in four months.

