 EnGeneIC said today that the FDA granted orphan drug designation to the company’s targeted EDV nanocells for the treatment of glioblastoma multiforme.
EnGeneIC said today that the FDA granted orphan drug designation to the company’s targeted EDV nanocells for the treatment of glioblastoma multiforme.
The clinical stage biopharmaceutical company is developing bacterially-derived EDV nanocells as a nanoparticle, siRNA and miRNA delivery platform designed to target and kill tumor cells, while triggering the immune system’s natural anti-tumor response.
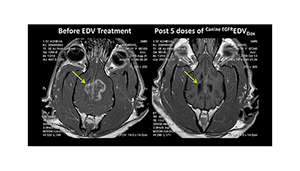
The EDV nanocells indicated for the treatment of glioblastoma multiforme are loaded with the chemotherapy agent doxorubicin.
Intravenously-injected EDV nanocells exit the vascular system attached to cancer cells using targeted bi-specific antibody, which EnGeneIC said helps to minimize toxicity.
Early clinical studies have shown promise for the EDV nanocell platform, according to EnGeneIC, and the company is planning future clinical trials in other cancer indications in Australia and the U.S.
“We are pleased that the FDA has granted Orphan Drug Designation to our targeted EDV nanocells for the treatment of GBM, a difficult-to-treat cancer indication with an especially poor prognosis,” joint-CEO and director Jennifer MacDiarmid said in prepared remarks. “This is not only an important U.S. regulatory milestone, but an exciting step towards our U.S. clinical advancement.”
