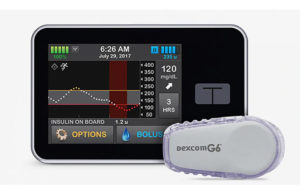
The FDA today cleared the Tandem Diabetes Care (NSDQ:TNDM) t:slim X2 insulin pump and Control-IQ advanced hybrid closed-loop technology to control Type 1 diabetes.
The agency also granted De Novo clearance to the Control-IQ device, which is compatible with a variety of continuous glucose monitors and insulin pumps.
The t:slim system, which also includes the recently approved Dexcom G6 CGM, is designed to help increase time in range (70-180 mg/dL), and is the first system cleared to deliver automatic correction boluses in addition to adjusting insulin to help prevent high and low blood sugar.
Diabetes advocates are hailing today’s FDA clearance and De Novo authorization as paving the way for a complete automated insulin dosing (AID) system — a true artificial pancreas. AID systems typically consist of a pump, a continuous glucose monitor (CGM) and software to control the system of compatible devices. Medtronic (NYSE:MDT) it won breakthrough status from the FDA for its investigational personalized closed-loop insulin pump system in February.
“Regulatory authorization of the Tandem Control-IQ algorithm for use as part of a hybrid closed-loop system is a huge win for the type 1 diabetes (T1D) community and a critical step forward in making day-to-day life better for people living with the disease,” said Aaron J. Kowalski, president & CEO of Type 1 diabetes research and advocacy organization JDRF. “This is the second hybrid closed-loop system on the market and the first algorithm authorized as an interoperable automated glycemic controller, which means it could be a component of an open-protocol system. People with different devices could use the algorithm and manage their glucose levels in a way that works best for them.”
The system integrates with Dexcom G6 continuous glucose monitoring (CGM), which requires no fingersticks for calibration or diabetes treatment decisions. Control-IQ technology for the t:slim X2 insulin pump is the first automated insulin dosing software in a new FDA interoperable automated glycemic controller category that automatically adjusts insulin delivery by connecting to an alternate controller-enabled insulin pump (ACE pump) and integrated continuous glucose monitor (iCGM), according to Tandem.
Control-IQ is the also first such controller that can be used with other diabetes devices that are designed to be integrated into a customizable for an automated insulin delivery system, the type of system that people with Type 1 diabetes and their families have been cobbling together themselves out of frustration with existing systems on the market.
Control-IQ was reviewed through the De Novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type. This is the third category classified by the FDA for the interoperability of devices as a complete automated insulin dosing (AID) system.
The Tandem system uses CGM values, in conjunction with other variables such as insulin on board, to predict glucose levels 30 minutes ahead and adjust insulin delivery accordingly, the company said. Control-IQ technology also offers optional settings for sleep and exercise that will change the treatment values to better match the different physiologic needs during these activities.
The t:slim X2 insulin pump was also the first to receive an ACE infusion pump classification in February 2019, and the first insulin pump designated as compatible with iCGM devices in June 2018.
“Not only do new closed-loop systems need to be effective at improving glycemic control, they must also be easy to understand and use so patients can experience the full benefits of the technology. The t:slim X2 insulin pump with Control-IQ technology successfully achieved both objectives in the clinical studies,” said Boris Kovatchev, director of the Center for Diabetes Technology at the University of Virginia and principal investigator of the International Diabetes Closed Loop (iDCL) trials, a recent National Institutes of Health-funded study found that a system controlled by the Tandem Control-IQ controller was more effective than existing treatments at controlling blood glucose levels in people with type 1 diabetes.
“With this clearance, we will be launching the most advanced automated insulin dosing system commercially available in the world today,” added Tandem Diabetes Care president & CEO John Sheridan in a news release. “This is a testament to our commitment to improving the lives of people with diabetes by offering simple-to-use products that deliver superior performance.”
“Today’s action continues the agency’s ongoing efforts to work with the diabetes community to help ensure the safety and efficacy of innovative and customizable diabetes management systems that may help patients better tailor their treatments to their individual needs,” said Dr. Tim Stenzel, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health in a news release. “The marketing authorization of this first stand-alone interoperable automated glycemic controller also allows substantially equivalent controller technologies that are developed for diabetes in the future to go through the 510(k) review process, helping to promote timely patient access to innovative technologies that can improve their care and quality of life.”
The NIH-funded trial randomly assigned 168 Type 1 patients ages 14 or older to either the Tandem device or treatment with the G6 CGM and another insulin pump that did not automatically adjust insulin. Patients in the Control-IQ arm increased the amount of time in glucose level range (70-180 mg/dL) by an average of 2.6 hours a day and showed improvements in time spent with high and low blood glucose, hemoglobin A1c and other measures compared to the control group.
No severe hypoglycemia events were observed in either group, but diabetic ketoacidosis occurred in a Control-IQ patient due to a problem with the equipment that delivered insulin. A previous problem with the Control-IQ’s handling of CGM data, which temporarily halted use of the software, was resolved in March.
Even though the system has been assessed for reliability, incorrect and inappropriate calculation, and command, delay of insulin delivery can still occur. Other risks associated with use of the interoperable controller can include incorrect insulin delivery as a result of loss of communication between connected devices, or from exploitation of cybersecurity vulnerabilities. These associated risks can lead to low blood glucose (hypoglycemia) or high blood glucose (hyperglycemia, including diabetic ketoacidosis).
With the authorization of the interoperable Control-IQ Technology controller and the establishment of some special controls, the FDA has created a new regulatory classification for this type of device. This means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process.
Shares in TNDM were up 0.96% to $58.24 in late-morning trading.
