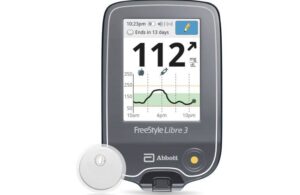
The FDA cleared Abbott’s next-generation FreeStyle Libre 3 in May 2022. Abbott designed FreeStyle Libre 3 as the smallest and thinnest CGM sensor in the world. The system constitutes the size of two stacked U.S. pennies. Users wear it inconspicuously on the back of the upper arm. It features an uncomplicated application process thanks to a one-piece applicator.
It recorded a 7.9% mean absolute relative difference (MARD). Abbott says the MARD measure of accuracy makes FreeStyle Libre 3 the most accurate 14-day CGM with readings sent directly to a smartphone every minute.
With a standalone reader, Abbott aims to get the FreeStyle Libre 3 added to Medicare’s list of covered systems “as soon as possible.” The Centers for Medicare & Medicaid Services (CMS) said expanded CGM coverage goes into effect this month.
“Our customers all over the world consistently tell us how our FreeStyle Libre technology has made an enormous, positive impact on their health and quality of life – they spend less time worrying and more time living,” said Jared Watkin, SVP for Abbott’s diabetes care business. “The FreeStyle Libre 3 reader provides more choice to people living with diabetes to have access to lifesaving technology that is smaller and easier to use and comes without the high-cost burdens of other systems.”
About the Abbott FreeStyle Libre 3 reader
Abbott’s FreeStyle Libre 3 reader is a small, handheld device. It displays real-time glucose readings directly from the small sensor worn on the back of the upper arm.
The system allows users to manage their diabetes quickly and easily by viewing glucose readings on a large, bright, easy-to-see screen. Users of the FreeStyle Libre 3 retain the option to use the current available smartphone apps.
Abbott’s reader uses a rechargeable lithium-ion battery. The system’s manual provides details on how to safely store, charge and use the device. That includes always using the Abbott-provided USB cable and power adapter.
Last week, Abbott issued a voluntary medical device correction to emphasize instructions for FreeStyle Libre continuous glucose monitor (CGM) readers. The company said it received a limited number of global reports (0.0017%) from users over several years saying their reader’s lithium-ion battery swelled or infrequently overheated. In very rare cases, users reported that the battery sparked or caught fire.
No readers are being physically recalled as a result of the warning. Customers can continue to safely use their readers with the Abbott-provided USB cable and power adapter. Customers do not need to return their readers.
An FDA notice issued shortly after offered more detail. It said the warning spans the FreeStyle Libre Flash, FreeStyle Libre 14-day and the FreeStyle Libre 2 Flash. All affected products are glucose monitoring systems. The notice does not affect any of the FreeStyle Libre family of sensors.
Abbott reported 206 incidents, including at least seven fires, one injury and no deaths involving this issue.
