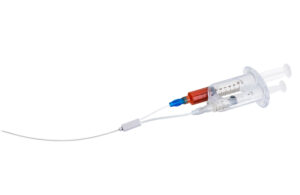
Kfar Saba, Israel–based VVT Medical says it specifically created ScleroSafe to provide efficient, non-thermal, non-tumescent treatment and management of varicosities in superficial veins. Its inverse-action dual syringe injects the ECA substance into the vein through one syringe while simultaneously aspirating blood and substance residuals through the second syringe. ScleroSafe accomplishes all of these actions within a single motion and with single-hand operation.
The company has already received approval for ScleroSafe in multiple regions, including Europe, Australia, Southeast Asia, and South America.
As many as 40 million people in the United States suffer from varicose veins, according to Beth Israel Deaconess Medical Center in Boston. Especially prevalent among women as they age, the veins often appear in the legs and pelvic area. They’re abnormal, dilated blood vessels that often look blue, bulging and twisted.
Sometimes, varicose veins are a minor inconvenience, but they can also lead to more serious problems as they become worse over time.
“ScleroSafe holds the promise of transforming varicose vein treatment, providing patients with a safe, effective, and straightforward option,” VVT Medical Erez Tetro said in a June 21 news release. “We firmly believe that our product has the ability to bring about a positive change in the lives of countless individuals across the United States and worldwide.”

