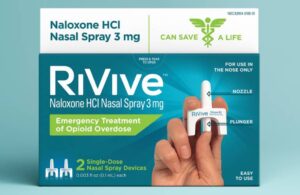
Pittsburgh-based HRT designed the 3 mg naloxone HCl nasal spray for the emergency treatment of opioid overdose. Approval helps make free or low-cost OTC nasal spray widely available in the U.S.
The FDA first approved Narcan 4 mg OTC naloxone nasal spray in March of this year. A different opioid overdose therapeutic, the Opvee nalmefene hydrochloride nasal spray from Opiant Pharmaceuticals, received approval in May.
RiVive uses an easy-to-use standard dose unit system. Each nasal spray device contains one dose. HRT plans to offer it exclusively in twin packs containing two single-dose devices.
“We are grateful that FDA granted RiVive approval so we can now achieve what most thought impossible and no other company has: broad delivery of a lower-cost nasal naloxone product without a prescription to save lives that could otherwise be lost to opioid overdose,” said Michael Hufford, co-founder and CEO of Harm Reduction Therapeutics.
As HRT is a non-profit pharmaceutical organization, no company, entity or individual will profit from RiVive sailes. The company plans to engage additional funding partners to make the affordable spray more accessible. HRT expects to make RiVive available in the U.S. in early 2024, primarily with harm reduction organizations and state governments.
The company said it plans to make at least 200,000 doses available for free. It wants to supply RiVive to communities that need it most.
RiVive’s manufacturing takes place under a commercial supply agreement with Catalent.
FDA has its say on another naloxone nasal spray approval
An FDA news release said it granted approval based on study data demonstrating similar levels of RiVive reaching the bloodstream compared to an approved prescription naloxone product.
The drug proved safe and effective for use as directed in its labeling. HRT also provided data demonstrating that consumers can understand how to use it safely and effectively without professional supervision.
“We know naloxone is a powerful tool to help quickly reverse the effects of opioids during an overdose. Ensuring naloxone is widely available, especially as an approved OTC product, makes a critical tool available to help protect public health,” said FDA Commissioner Dr. Robert M. Califf. “The agency has long prioritized access to naloxone products, and we welcome manufacturers of other naloxone products to discuss potential nonprescription development programs with the FDA.”

