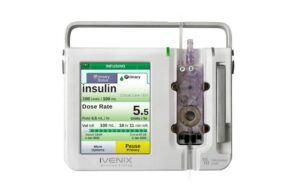
This recall affects the LVP-0004 model of the Ivenix large-volume pumps. Fresenius Kabi distributed these systems between Oct. 1, 2021, and July 31, 2023. In total, the company recalled 938 devices in the U.S. since initiating the recall on Nov. 29, 2023.
Fresenius Kabi reports no injuries or deaths related to the recall, according to the FDA.
The company recalled the pump because some units have mechanical issues with the fluid valve pins. This issue may cause the pins — located inside the pump’s internal housing — to not move properly. Instead of going where they should, the pins could impact the side of a sensor.
When this occurs, the system detects the failure and sets off an alarm, stopping an infusion if in progress and preventing the pump from working. The failure may also be detected during pump setup, which can cause a delay in starting treatment.
Using the affected pumps could lead to underdosing, interruption in therapy or delay in therapy. These could all lead to serious harm or death.
Fresenius Kabi’s Ivenix large-volume pump is one of three primary components of the Ivenix Infusion System. The pump uses air pressure to precisely control the flow of fluids to patients in hospitals and outpatient centers. It only has compatibility with specific sterile, single-use, disposable administration sets.
Fresenius Kabi suggested that affected customers increase their clinical monitoring if the pump is being used to deliver life-sustaining medications. They should use a different pump if the pump exhibits the issue during setup. Users should also remove pumps that exhibit a problem alarm during use.

