 Inovio Pharmaceuticals (NSDQ:INO) said today that it priced its underwritten public offering of 12.5 million shares of common stock at $6.00 apiece. The offering is expected to bring in nearly $75 million for the Plymouth Meeting, Penn.-based company.
Inovio Pharmaceuticals (NSDQ:INO) said today that it priced its underwritten public offering of 12.5 million shares of common stock at $6.00 apiece. The offering is expected to bring in nearly $75 million for the Plymouth Meeting, Penn.-based company.
The offering, which is expected to close on July 25, includes a 30-day option for underwriters to buy up to 1,875,000 additional shares of common stock.
Inovio is slated to use its newly-acquired funds to support general corporate purposes, including clinical trial expenses and its R&D efforts.
Last month, the company announced that it is launching a late-stage trial of its investigational DNA immunotherapy candidate designed to treat cervical dysplasia caused by human papillomavirus.
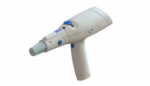
The moves came after the FDA lifted a hold that it placed on the clinical program in October last year. At the time, the FDA requested data to support the shelf-life of the disposable parts of Inovio’s Cellectra electroporation device.
Inovio plans to evaluate the efficacy of VGX-3100, its first immunotherapy, in regressing cervical high-grade squamous intraepithelial lesions – a direct precursor to vervical cancer – and its ability to eliminate the HPV infection that causes the lesions. Data from the pivotal trial will support the potential licensure of VGX-3100 as the 1st immunotherapy for this disease, according to Inovio.
The company’s plasmid DNA immunotherapy is injected intramuscularly, followed by electroporation using Inovio’s Cellectra delivery device. Cellectra uses a pulse of electricity to briefly open the pores in a cell’s membrane and introduce the DNA.

