![The Inlexzo system. [Image courtesy of Johnson & Johnson]](https://www.drugdeliverybusiness.com/wp-content/uploads/2025/09/Inlexzo-JJ-2-1-300x195.jpg)
The intravesical gemcitabine-releasing system, previously known as TAR-200, treats patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle invasive bladder cancer (HR-NMIBC) with carcinoma in situ (CIS), with or without papillary tumors.
A healthcare professional places TAR-200 into the bladder using a co-packaged urinary placement catheter. The procedure takes place in an outpatient setting in less than five minutes and requires no general anesthesia or further monitoring. Inlexzo remains in the bladder for three weeks per treatment cycle for up to 14 cycles.
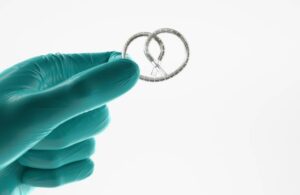
J&J calls Inlexzo a potentially practice-changing approach for treating certain types of bladder cancer. The FDA granted priority review to a new drug application (NDA) for the system earlier this year.
The Phase 2b SunRISe-1 study, which demonstrated an 82.4% complete response rate, supported the FDA approval. More than half (52.9%) of patients remained cancer-free at least one year or more after achieving a complete response. J&J noted that the majority of adverse reactions proved mild and moderate.
“We are proud of the science that has brought us to this historic moment,” said Dr. John Reed, EVP, R&D, Innovative Medicine, J&J. “Inlexzo is a novel therapy with powerful efficacy and demonstrated safety profile. As the only major healthcare company that hosts both pharmaceuticals and medical devices, we leveraged the speed and scale of Johnson & Johnson to accelerate innovation and deliver this important therapy to patients.”
