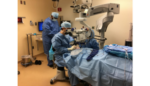
Massachusetts Eye and Ear Infirmary made history this week, becoming the first institution to use an FDA-approved gene therapy to treat a patient with an inherited disease.
Spark Therapeutics‘ (NSDQ:ONCE) Luxturna gene therapy, which was approved by the FDA in December, is designed to improve vision in patients with inherited retinal disease caused by a particular genetic mutation. Yesterday, Dr. Jason Comander injected the treatment into the eye of a 13-year-old boy from New Jersey.
“It is an honor to be involved in this exciting procedure following FDA approval, and to play a role in allowing a 13-year-old boy an opportunity to enjoy improved vision for years to come. This project has been underway for 20 years, and the early successes with this therapy demonstrated by Jean Bennett, Al Maguire, and others, inspired me to dedicate my career toward helping patients with inherited retinal diseases” Comander said in prepared remarks. “I am so excited the time has come when we can offer this groundbreaking therapy to our patients, who are truly in need of our help.”
People with mutations in the RPE65 gene do not produce a protein that is needed for the retina to function. Luxturna is a modified virus that doctors can inject into a patient’s eyes, where it delivers a healthy copy of the dysfunctional gene.
“Today we celebrate the decades of work by many individuals to bring gene therapy from science fiction to clinical reality for patients with inherited retinal disease,” Dr. Joan Miller, chief of ophthalmology at Mass. Eye and Ear and Mass General Hospital, added. “Our hope is that our ophthalmology community can leverage Luxturna’s success to accelerate the development of similar gene therapies for the many blinding retinal diseases that still afflict our patients.”
Spark’s therapy, which proved effective in clinical trials, comes with a hefty price tag – $850,000 for both eyes.

