Data came from a subset of 3,211 pediatric and adolescent patients (15 years old and below) with type 1 diabetes using the MiniMed 780G system with the Guardian Sensor 3 in Europe.
According to a news release, the average time in range of 74% surpassed clinical consensus guidelines and closely mirrored the system’s time in range for adults (77%). In addition, overnight time in range of 82% matched that of adults for the MiniMed 780G advanced hybrid closed loop (AHCL) system that offers near-real-time basal insulin and auto-correction boluses every five minutes.
Fridley, Minnesota–based Medtronic collected aggregated information from children under 15 whose caregivers agreed to provide anonymized data automatically uploaded to the CareLink Personal platform from Aug. 27, 2020, to July 22, 2021.
Medtronic said that a large majority of the users in the analysis are achieving glycemic goals recommended by major diabetes professional organizations. According to the company, 75.3% of users had a glucose management indicator (GMI) of less than 7%, while 69.6% had a time in range above 70% and 67.5% of pediatric users achieved both a GMI under 7% and time in range above 70%.
Medtronic’s sub-analysis of 661 patients 15 years old and under with at least 10 days of continuous glucose monitoring (CGM) data both pre- and post-AHCL initiation observed substantial improvements across both time in range and GMI with a 12% increase in time in range to 74% on average. Patients were able to stay in AHCL mode 93% of the time once it was initiated.
CMO of Medtronic’s diabetes business, Dr. Robert Vigersky, noted that young adults around age 15 have the highest reported A1C in the type 1 diabetes exchange registry (which holds data for over 31,000 individuals), demonstrating the challenge in diabetes management in the younger population.
“These results are extremely encouraging. Glycemic control has been much harder to achieve in children due to unpredictable factors common in this age group, including physical growth and development, hormonal changes and active lifestyles. … Because the algorithm in the MiniMed 780G system adjusts basal and correction insulin doses in near real-time every 5 minutes thereby providing near real-time course correction, it helps make up for underestimated carbohydrate counting and occasional late or missed meal doses,” Vigersky said in the release. “The Medtronic AHCL algorithm offers advanced protection and permits unprecedented personalization in insulin delivery by offering a wide range of active insulin time settings and three different glucose targets.
“These improvements reinforce that the MiniMed 780G system is a better alternative than previous therapy these patients were on, even for those who were relatively well-controlled.”
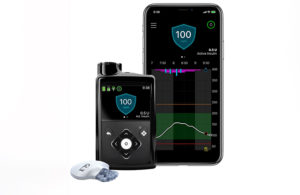
MiniMed 780G is Medtronic’s most advanced insulin pump system and is approved for treating type 1 diabetes in users between age 7 and 80 years old. The pump is available in 38 countries across Europe, the Middle East and Africa as the FDA reviews it for approval in the U.S.
“We know that adolescents in particular lead very active lives and often eat on the go — running from one activity to the next. This system was designed to help individuals living with diabetes have some extra coverage and protection when life gets in the way and they’re not able to manage their diabetes in the way they’d like to,” Medtronic diabetes VP of customer experience Julie Foster said. “We’re confident we’ve designed a system that keeps lifestyle and experience front and center as we work to help make life easier for people living with diabetes.”
Medtronic is making waves in the diabetes care space for multiple reasons as, earlier this week, reports in Israel claimed that the company is in advanced negotiations to acquire insulin pump maker Triple Jump for $300 million. Additionally, last month, the company purchased intellectual property relating to implanted infusion pumps from the Alfred E. Mann Foundation for Scientific Research (AMF). The deal will see AMF work with Medtronic to develop the technology into a next-generation implantable insulin pump for people with hard-to-treat type 1 diabetes.
The company has struggled with previous iterations of the MiniMed insulin pump platform, having earlier this month expanded the FDA Class I recall of its MiniMed 600 series pumps, which has led to a total of 463,464 devices recalled in the U.S.

