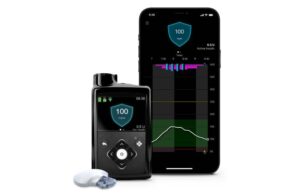
MiniMed 780G offers automated insulin delivery to those with type 1 diabetes. It features SmartGuard technology to eliminate the need for fingersticks. Its Meal Detection Technology provides automatic adjustments and corrections to sugar levels every five minutes.
The urgent field safety notice distributed by Medtronic is dated May 2024.
Medtronic said in the notice that some MiniMed 780G customers received replacement battery caps not approved for their pump. Those battery caps (ACC-1527) come in smaller in size than those approved for their specific pump model (ACC-1527).
When used, the incorrect battery cap could fail to securely seal the battery compartment. This could allow for moisture ingress and damage to the pump electronics. When the pump detects no battery source, it triggers an “insert battery” alarm, stopping insulin delivery immediately. After 10 minutes, the alarm sound increases to a siren and the pump turns off.
Pump and insulin delivery stoppages due to power loss can lead to varying levels of high blood sugar, including diabetic ketoacidosis (DKA). As of Nov. 28, 2023, Medtronic received four complaints from customers using the battery cap not approved for their pump. No complaints included an allegation of harm to the user related to the issue.
Medtronic instructed users to examine which model battery cap they have to identify which holds approval for their pump. A diagram provided by the company highlights the differences:


