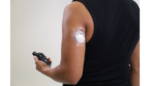
 The FDA has cleared Medtronic‘s (NYSE:MDT) Guardian sensor, part of the medtech titan’s MiniMed 670G automated insulin delivery system, to be worn on the upper arm.
The FDA has cleared Medtronic‘s (NYSE:MDT) Guardian sensor, part of the medtech titan’s MiniMed 670G automated insulin delivery system, to be worn on the upper arm.
The device is Medtronic’s newest and most accurate continuous glucose monitor, the company touted, and is the only sensor approved by the FDA for use with a hybrid-closed loop system to control insulin delivery.
“The performance of the Guardian Sensor 3 has been extremely impressive and this new arm indication now offers added convenience and flexibility for my patients who like to have as many options as possible to address their personal needs,” Dr. Bruce Bode, a specialist with Atlanta Diabetes Associates and a professor at Emory University, said in prepared remarks.
“These continued enhancements demonstrate a keen focus on the part of Medtronic to deliver a positive patient experience in addition to optimizing outcomes through technological advancements – that can prove to be just as meaningful for quality of life.”
Medtronic’s MiniMed 670G system is designed for people with Type I diabetes ages 14 years and up. The system uses Medtronic’s SmartGuard algorithm to automatically adjust insulin delivery every five minutes based on data provided by the Guardian Sensor 3.
“We are steadfast in our commitment to delivering both best-in-class clinical outcomes as well as best-in-class experiences for our customers,” Alejandro Galindo, president of the intensive insulin management division in Medtronic’s diabetes business, added.
“We are inspired by the many voices in the diabetes community who continue to shape our offerings in a meaningful way as we work to address the wide spectrum of needs within the community. We are very pleased to offer this added flexibility for those who rely on our therapies for their diabetes management needs.”

