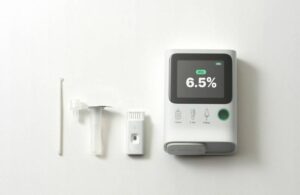
Seoul, South Korea–based Orange Biomed operates out of Research Triangle Park, North Carolina in the U.S. The company currently has ongoing studies for its OBM rapid A1c test. It designed the glycated hemoglobin analyzer to function through single-cell analysis of red blood cells.
Co-founder Yeaseul Park describes OBM rapid A1c as a portable, guided, five-minute test. It requires only a single drop of blood to produce accurate results. The system integrates lab-level technology into portable devices, making healthcare accessible, accurate and affordable.
Orange Biomed featured in Drug Delivery Business News’ 11 diabetes startups you need to know. The company plans to unveil its latest technology at the 83rd Scientific Sessions of the American Diabetes Association (ADA) in June.
“Current technologies are likely to produce inaccurate A1c results disproportionately more for non-white patients due to hemoglobin variants,” said Park. “Hemoglobin variants are more common in patients with African, South and Southeast Asian, and Mediterranean ancestry. Inaccurate results can delay diabetes diagnosis and serious long-term health damage. We invented a new method of A1c testing, free from hemoglobin variant interference, to facilitate earlier detection and management of diabetes across all patient populations.”
In pre-Series A funding rounds, Orange Biomed brought in more than $2 million in investments. It currently has OBM rapid A1c undergoing global studies with Asan Medical Center. The company aims to garner FDA clearance within one year.
