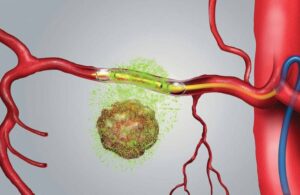
Los Altos, California-based RenovoRx develops precision oncology therapies based on a local drug delivery platform. Closing this funding round extends the company’s cash runway as it conducts its pivotal TIGer-PaC Phase III clinical trial. It expects the funds to aid operations through 2024.
TIGer-PaC uses RenovoRx’s proprietary TAMP therapy platform to evaluate its first product candidate, the RenovoGem. RenovoGem, a novel oncology drug-device combination, is designed to treat locally advanced pancreatic cancer (LAPC). The study compares TAMP treatment to the current standard of care, systemic intravenous chemotherapy.
RenovoRx also plans to use funding to engage additional clinical trial sites in preparation for its Phase II CouGar study in bile duct cancer. That study also looks at RenovoGem in a difficult-to-access solid tumor cancer. The company also identified pipeline applications such as non-small cell lung cancer, uterine tumors, glioblastoma and sarcoma. It expects to require additional funding to pursue those opportunities.
The funding round saw RenovoRx sell an aggregate of more than 6.1 million shares of common stock and warrants. Non-company insiders investing paid 99¢ per share. Company management and directors purchased shares at $1.22 per share. The company says this reflects their strong belief in RenovoRx.
“We are thrilled to start the new year with the closing of this important financing, especially in a challenging market climate,” said Shaun Bagai, RenovoRx CEO. “The net proceeds bolster our balance sheet and will allow us to drive our pivotal trial towards what we hope will be another positive interim analysis by the end of this year.”
