Research presented at the Society of Interventional Radiology Annual Scientific Meeting in Boston supported the hydrogel, which was injected into spinal discs in an effort to relieve chronic low back pain caused by degenerative disc disease (DDD).
According to a news release, hydrogels have been used for a number of years to treat DDD, but the current study is the first of this particular gel — called Hydrafil — in humans. The second-generation hydrogel, developed by ReGelTec, received FDA breakthrough device designation in 2020.
Six-month results from the small study of 20 patients culminated in all 20 reporting significantly less low back pain, declining from an average self-reported pain level of 7.1 (on a scale of 1-10) down to 2.0. Other experiences included improved physical function, with average scores falling from 48 to six on a questionnaire to gauge the impact of low back pain in preventing patients from the ability to perform normal activities.
“If these findings are confirmed in further research, this procedure may be a very promising treatment for chronic low back pain in those who’ve found insufficient relief from conservative care,” the study’s lead author, Dr. Douglas P. Beall, chief of radiology services at Clinical Radiology of Oklahoma and medical advisor to ReGelTec said in the release. “The gel is easy to administer, requires no open surgery, and is an easy procedure for the patient.”
The study’s patient population ranged from age 22 to 69, all with chronic DDD low back pain. Each described their pain as four or higher on the 10-point scale and none had found more than mild relief from conservative care, which includes rest, analgesics, physical therapy, and back braces.
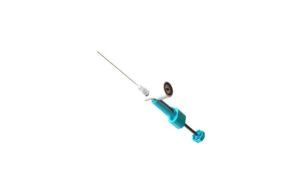
Patients were sedated for the procedure and the gel was heated to become a thick liquid before researchers, guided by fluoroscopic imaging, used a 17-gauge needle to inject the gel directly into the affected discs. The gel then filled in cracks and tears, adhering to the disc’s center and outer layer.
“We really have no good treatments for degenerative disc disease, aside from conservative care,” Beall said. “Surgery is statistically no more effective than conservative care and can potentially make things worse; nerve ablation is appropriate for only a few patients; and existing hydrogels are inserted through an incision as a soft solid, which can pop out of place if you’re not highly skilled in placing it.”
“Because this gel is injectable, it requires no incision, and it augments the whole disc, restoring its structural integrity, which nothing we have currently can do.”
