Ribonucleic acids, which can alter the behavior of cancerous cells, can be effectively ferried to tumors using a human protein called albumin, according to research conducted by a team of engineers at Vanderbilt University.
The team’s work could help boost treatment for patients with triple-negative breast cancer, the researchers reported in the journal Proceedings of the National Academy of Sciences.
The researchers modified a small-interfering ribonucleic acid molecule, siRNA-L2, so that it could fit into an albumin pocket that usually transfers fatty acids around the body. They compared their ribonucleic acid-albumin combo against jetPEI nanoparticles – a commonly-used synthetic carrier.
The team observed that siRNA-L2 had no apparent dose-limiting toxicity, meaning that a high dose of cancer-killing drug could be delivered to a tumor while limiting harm to the patient.
“We used albumin because it’s the highest-concentrated protein in your blood,” principal investigator Craig Duvall said in prepared remarks. “Our molecule, siRNA-L2, binds into the fatty acid pocket of albumin. If we put siRNA directly into the body without a carrier, it’s cleared out by the kidneys in two minutes. If we load siRNA into synthetic nanoparticles to avoid that, then they’re filtered out by the liver. Albumin circulates in the body for days, making the siRNA-L2 molecules more available for delivery into tumors.”
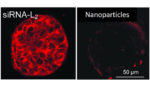
The researchers reported that 100% of tumor cells tested positive for siRNA-L2 compared to just 60% when the molecule was ferried by jetPEI. After the molecule arrives at the tumor, it reportedly silences a gene linked to the tumor’s growth and survival.
In collaboration with the Vanderbilt University Medical Center, the team tested siRNA-L2 in human breast cancer tissue taken from a donor.
According to the team, the siRNA-L2 molecule was three times as prevalent in the tumor than siRNA delivered using synthetic nanoparticles.
“What fascinates and excites me most about this approach, in addition to improved tumor penetration, is lack of toxicity at a relatively high dose,” cancer biologist Dana Brantley-Sieders added. “We could potentially use our siRNA delivery system to target several genes simultaneously or sequentially. Most cancers are driven by multiple abnormal genes, so targeting one often leads to activation of others as the tumor adapts.”

