The research found immediate and sustained improvements for a diverse range of subjects using Control-IQ technology, according to the research published in the journal Diabetes Technology & Therapeutics. There was an average increase in time in range of 2.8 hours and a reduction in hemoglobin A1c compared to control groups in people ages 2 to 72 years old.
“Control-IQ technology delivered the most robust improvements in those entering the study with the highest hemoglobin A1c and lowest time in range,” Dr. Roy W. Beck, executive director of the Jaeb Center for Health Research (Tampa, Florida), said in a news release. “The high number of automatic boluses given by the system in this group likely reflect previously missed meal boluses or lack of manual correction boluses when on conventional therapy and demonstrates the substantial impact Control-IQ technology’s auto-bolusing feature can have for people struggling on a standard pump or multiple daily injections.”
Boris Kovatchev, director of the Center for Diabetes Technology at the University of Virginia, noted that the study found Control-IQ benefits regardless of age, ethnicity, education, or previous pump experience. “It is clear from these results, which are consistent with real-life data from thousands of current Control-IQ technology users, that this technology should be considered as an option for anyone living with type 1 diabetes.”
Dr. Jordan Pinsker, VP and medical director at Tandem Diabetes Care, said the three randomized, controlled trials have provided Control-IQ with the most robust data set supporting its benefits compared to any other automated insulin delivery system that is presently out there.
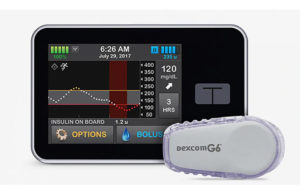
Tandem’s Control-IQ technology automatically observes and manages blood glucose levels. This artificial pancreas employs an insulin pump that uses sophisticated algorithms based on glucose monitoring data from the Dexcom G6 CGM, adjusting insulin dosage as required. The FDA has cleared Control-IQ for use in individuals aged 6 and older with type 1 diabetes.
