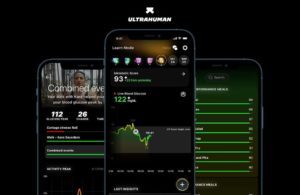
Bangalore, India-based Ultrahuman develops the Ultrahuman Cyborg, a continuous metabolism tracker designed to help people with diabetes track blood glucose in real-time and optimize their diet and exercise.
The platform goes beyond traditional markers like body mass index, weight and point-in-time blood tests, utilizing biomarkers to tell users how their body reacts to a certain type of food, how to fuel efficiently for exercise and how to eat for better sleep.
“Our mission is ‘to help people optimize their health by providing them access to deeply personalized insights and real-time nudges,'” the company wrote in a news release.
Ultrahuman said the funds raised in the Series B will give the company the ability to launch in new geographies, strengthen its teams and improve its life sciences research capabilities.
Alpha Wave Incubation (AWI), which is backed by DisruptAD and managed by Falcon Edge, Steadview Capital, Nexus Venture Partners, Blume Ventures and Utsav Somani’s iSeed fund all contributed to the financing. Additionally, angel investors, including Tiger Global’s Scott Schleifer, Sandeep Singhal, Kunal Shah, Sujeet Kumar, Deepinder Goyal, Gunjan Patidar, Gaurav Munjal, Revant Bhate, Mohit Gupta, Vikram Dhingra and Roman Saini also participated.
