 In January last year, the National Institutes of Health issued a new requirement for grant funding in basic science: Researchers must discuss how gender as a biological variable will impact their research.
In January last year, the National Institutes of Health issued a new requirement for grant funding in basic science: Researchers must discuss how gender as a biological variable will impact their research.
Teresa Woodruff, director of the Women’s Health Research Institute at Northwestern University, told Drug Delivery Business News that this helped support their effort to develop a model that could eventually serve as a way for pharmaceutical companies to consider female biology when developing drugs.
Woodruff, researchers at the University of Illinois at Chicago and Draper Laboratory worked collaboratively on an NIH-funded project to make a 3D model of the female 28-day menstrual cycle using human tissue. The team’s work was published today in Nature Communications.
“I think pharma will quickly adapt this and put this as part of the chain of command that they’re identifying next generation drugs,” Woodruff said.
Drug-makers use in vitro preclinical tests to evaluate drugs for their safety and efficacy on a cellular level, but biomedical engineers at Draper pointed out that these models don’t account for the hormonal effects of female biology.
“There’s been a push from the NIH to incorporate female biology into the drug development process, and I think its starting to happen, certainly in animals, but they just haven’t developed the in vitro model yet,” Jeffrey Borenstein said. “There isn’t currently a similar model big pharma uses at this point.”
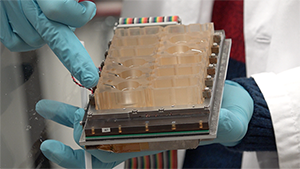
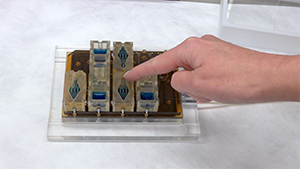 The microfluidic device, called Evatar, is the size of a smart tablet, Draper’s Jonathan Coppeta explained, and has 12 sub-sections that house human tissue and create a micro-environment for the tissue to behave as it would in the human body. Evatar includes 3D models of ovaries, fallopian tubes, the uterus, cervix, and liver, with Draper’s micro-pump moving a universal fluid throughout all of them, mimicking the effects of blood.
The microfluidic device, called Evatar, is the size of a smart tablet, Draper’s Jonathan Coppeta explained, and has 12 sub-sections that house human tissue and create a micro-environment for the tissue to behave as it would in the human body. Evatar includes 3D models of ovaries, fallopian tubes, the uterus, cervix, and liver, with Draper’s micro-pump moving a universal fluid throughout all of them, mimicking the effects of blood.
In pre-clinical models, researchers traditionally culture cells in flat, plastic wells. Woodruff argued that in those types of tests, the cells are sitting in “metabolic waste”, so researchers are actually observing cell signaling as it relates to stress. Instead, their model uses microfluidics to simulate the environment of the human body and observe the cells’ true reactions.
“I think in the broadest sense, having microfluidics systems that enable us to go from static to dynamic cultures is really going to revolutionize cell biology,” she said.
Moving fluid around an “organ-on-a-chip” is no easy accomplishment – it needs to be done accurately and reliably. Borenstein said that Draper’s micro-pump was initially designed for a separate drug delivery project that the company was working on in collaboration with Massachusetts Eye & Ear Infirmary. The pump they developed is very small, reliable and low power, according to the team.
Most importantly, the team needed an actuator that could pump fluid reproducibly over millions of cycles – including the 28-day cycle characteristic of the female reproductive tract.
“We had this idea to take micro-pumps that we developed for implants and integrate that into the well plate. That’s enabled us to create a more portable and modular kind of technology,” Borenstein said.
Woodruff’s team developed the media that flows through the model, using salts, amino acids, insulin and other factors, and hormones.
“Traditionally, everyone who studies different organs has their own special cocktail,” Woodruff explained. “If you’re going to start linking systems together, we need to have 1 way that those cells all communicate.”
The research is a part of the NIH’s larger effort to model an entire human body “on-a-chip”.
“There’s a vision that these human organ systems, whether they be the reproductive model we’ve developed with Dr. Woodruff or any of these other models can provide a much better indicator of the efficacy of the drugs before they go into clinical trials,” Borenstein said. “There is always discussion about whether these systems would replace all or part of the animal studies, but I think either way they would greatly increase the accuracy of the pre-clinical evaluation process.”
“I don’t think there will be 1 model that captures the entire human physiology,” Coppeta said, but Draper is working with other companies, including Pfizer (NYSE:PFE), to develop a variety of organ models to be used as dynamic testing-grounds for drugs.
The group predicted that this type of model could 1 day be used with a patient’s own stem cells to create a personalized model and test to see how a patient would react to a particular drug.
“This is really a 1st step towards developing new technologies that can be broadly applicable to not only reproductive cells, but systemic biology and health,” Woodruff said.

