Manufacturing automation is crucial to achieving the precision, quality, and volume required in the medical device industry. Advanced robotics, AI, modular designs, and wireless technology offer the opportunity for dramatic improvements in efficiency and quality control, but leveraging these new technologies requires specific expertise. Most Medtech companies specialize in bringing life-changing products to market, not […]
Patient Training is Broken – And Everyone is Paying For It
Noble International is working with Pharma and Specialty Pharmacy to fix the problem. The report, “The Current Paradigm for Biologic Initiation: A Mixed-Methods Exploration of Practices, Unmet Needs, and Innovation Opportunities in Self-Injection Training,” chronicles a study meant to examine the current injection initiation experiences of patients. Here, significant gaps in training and support were […]
Noble Leads with its User-Centered Design Strategy
By Tim McLeroy, Executive Director of Marketing and Patient Services, Noble International At Noble, the patient journey drives everything we do. Our patient-centered approach pushes us to fully understand and characterize patient needs, providing data and experiences that help us ease the burden and anxiety of self-administered treatments. While drug delivery devices are well designed […]
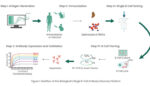
Discovering Antigen-specific Monoclonal Antibodies using Single B Cell Screening
Introduction Since the approval of Orthoclone OKT3 in 1986, more than 100 monoclonal antibodies (mAbs) have been approved by the Food and Drug Administration (FDA) to treat a variety of diseases ranging from autoimmune disorders, infectious diseases, and cancer [1-2]. In the context of the current COVID-19 pandemic, it’s crucial that these biologics are developed […]
Change Can Be Challenging: Especially for Patients
Noble Can Help Alleviate Patient Anxieties Caused by Switching to Biosimilar Therapies Change can be difficult for patients, particularly when embarking on a new treatment plan. Patients who require treatments for a variety of chronic illnesses—ranging from diabetes and rheumatoid arthritis to multiple sclerosis, Crohn’s disease and cancer— are increasingly prescribed biosimilar therapies to self-inject […]
Noble’s AdhereIT® Provides Flexibility of Design, Empowers Patients and Encourages Adherence
A recent study1 commissioned by Noble International analyzed the biologic initiation process and current gaps pertaining to patient guidance and self-injection training practices. Anecdotal evidence showed that more than one-third of participating patients lacked the necessary confidence to self-inject during their first six months of treatment due to inadequate training practices. The study further maintained […]
A new way to access scientific papers?
Losing university access doesn’t mean losing access to articles anymore By DeepDyve Digital Library Team Many researchers are surprised to find that leaving university also means leaving behind something they didn’t expect—easy access to peer-reviewed literature. Especially for those who have gone to work at startups or other smaller organizations, finding a way to do […]
Paradigm Shift: How Noble Can Help Patients Reduce Errors During Biologic Initiation
Unmet Training Needs For Patients Using Self-Injectable Drug Therapies Comes At A High Cost A recent report published in Expert Opinion On Drug Delivery uncovered a startling paradigm regarding unmet training needs for patients who begin self-injectable therapies for chronic disease management. The authors of the report, “The current paradigm for biologic initiation: a mixed-methods […]
What needle should you use?
By Nathan Swihart, Social Media and Internet Sales Specialist Every professional should have comprehensive knowledge on the products they use on a daily basis. One of the widest-used and most fundamental tools throughout the history of the medical industry is the hypodermic needle. Whether you work in the laboratory, pharmaceutical, veterinary, or human healthcare fields, […]
Noble can help patients reduce errors when self-administering biosimilar therapies
In today’s evolving healthcare environment, it’s not surprising that biosimilars have become more widely prescribed by physicians as an affordable alternative to biologics, which have been estimated to account for 38-40% of all pharmaceutical spending and average more than $100,000 per patient per year.1 Following this trend, in 2021, the biosimilar global market size was […]