 Spanish researchers demonstrated that bacterial inclusion bodies, non-toxic nanostructures, hold promise for a sustained, local delivery of protein drugs by mimicking components of a mammal’s endocrine system.
Spanish researchers demonstrated that bacterial inclusion bodies, non-toxic nanostructures, hold promise for a sustained, local delivery of protein drugs by mimicking components of a mammal’s endocrine system.
New materials developed as controllable, localized systems of drug delivery often aim to be immobilized in a given tissue as a drug “depot” for long term supply of the drug. Hydrogels, polymers, and other biomaterials have been researched as scaffolds for drug delivery, but toxicity and rejection can present issues in the system’s long-term stability.
The team of researchers turned to the endocrine system, which uses secretory granules to accumulate functional proteins for long-term storage. In response to particular stimuli, the reservoirs will release their hormones. Bacterial inclusion bodies can mimic the function of secretory granules; they are mechanically stable and can retain active protein for localized delivery of a therapeutic protein. Previous work shows that the bacterial nanostructures can successfully penetrate mammalian cells.
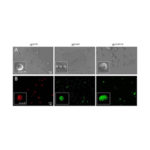
Researchers evaluated the bacterial inclusion bodies as a potential delivery system for a protein that induces apoptosis, or cell death, upon local delivery to targeted tumor tissues, according to a study published today in Nature.
They determined that local injection was the best delivery method of the nanostructures. Upon injection of the bacterial inclusion bodies, the team observed high stability and low toxicity. The nanostructures also successfully induced apoptosis in the targeted tissue.
The team concluded that bacterial inclusion bodies can mimic the secretory granules of the endocrine system and behave as depots of functional proteins upon their immobilization by local injection.

