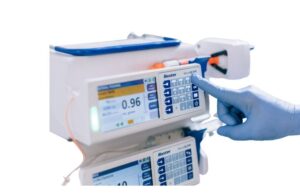
Baxter (NYSE:BAX) issued an urgent medical device correction notice regarding its Novum IQ syringe infusion pump.
A few weeks ago, the FDA deemed a Novum IQ recall Class I because the pump may incorrectly indicate the completion of an infusion.
Deerfield, Illinois-based Baxter notified customers of the latest issue in October. This correction comes as a result of the potential for an incomplete infusion following one or more downstream occlusion alarms. The company has work underway on a software upgrade and has not received reports of serious injury related to the issue.
Baxter said in a news release that, after one or more downstream occlusion alarms, the pump may display an “Infusion Complete” alarm. However, this alarm can occur despite remaining fluid in the syringe, potentially leading to underdosing or therapy interruption. A patient who fails to receive the intended dose of a prescribed medication could face serious or critical adverse health consequences.
Affected customers can continue using Novum IQ pumps until Baxter makes the software update available. They must follow reinforced guidelines, including choosing the smallest compatible syringe size necessary. Prior to infusion, they should ensure an appropriate downstream occlusion pressure setting. Users should continue monitoring the “Volume to be Infused” setting and the volume delivered during therapy.
The notice applies to Novum IQ syringe infusion pumps with the product code 40800BAXUS.

