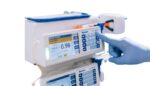
Baxter (NYSE:BAX) announced today that the FDA granted 510(k) clearance for its Novum IQ large-volume infusion pump (LVP) with Dose IQ safety software. Clearance adds the LVP to the Novum IQ platform, which already includes the Baxter syringe infusion pump (SYR). It enables clinicians to utilize a single, integrated system across a variety of patient […]
Baxter
Baxter issues Novum IQ infusion pump warning
Correction: the original version of this article identified this as another recall for Novum IQ. Baxter issued a notification related to a Nov. 15 recall, not a notification for a separate recall. This story has been updated. Baxter (NYSE:BAX) issued an urgent medical device correction notice regarding its Novum IQ syringe infusion pump. A few […]
Baxter recalls some syringe pumps due to underdosing risk
The FDA determined that the recall of the Baxter (NYSE:BAX) Novum IQ syringe pump is Class 1, the most serious kind. Baxter designed its Novum IQ syringe pump as an infusion pump to deliver fluids into a patient’s body in a controlled manner. The device is suitable for patient care in hospitals and outpatient facilities […]
Baxter warns on some infusion pumps due to potential false alarms
Baxter (NYSE:BAX) issued an urgent medical device correction for some Spectrum V8 and Spectrum IV infusion pumps in the U.S. The FDA later issued a notice identifying the recall as Class I, the most serious kind. Baxter recalled 22,769 devices in total. Affected pumps in the U.S. and Puerto Rico received upgrades to software versions […]
Recall of Baxter Clearlink due to risk of leaks is Class I
The FDA announced today that the recall of the Clearlink basic solution set with Duovent from Baxter (NYSE:BAX) is Class I, the most serious kind. Clearkink with Duovent is part of a system for administering drugs and solutions to patients. Health providers use the majority of the sets to deliver hazardous drugs, such as chemotherapy […]
CISA warns on cybersecurity vulnerabilities for certain Baxter infusion pumps
The U.S. Cybersecurity and Infrastructure Security Agency (CISA) today issued a warning on some Baxter (NYSE:BAX) infusion pumps. Sigma and Baxter Spectrum infusion pumps are included in a CISA notice over remotely exploitable vulnerabilities. Those vulnerabilities include: missing description of sensitive data, use of externally controlled format string and missing authentication for critical functions. The […]
FDA clears syringe infusion pump with Dose IQ software from Baxter
Baxter (NYSE:BAX) announced today that the FDA issued 510(k) clearance to its Novum IQ infusion pump with Dose IQ safety software. Deerfield, Illinois-based Baxter designed its new Novum IQ syringe infusion pump (SYR) to deliver small amounts of fluid at low rates. It includes a technologically integrated user experience with enhanced safety features. The system […]
BD wins patent fight against Baxter over drug-transfer system
BD (NYSE:BD) picked up a legal victory in a patent spat with Baxter (NYSE:BAX) over a system for moving drugs. A federal judge in Chicago ruled that BD’s PhaSeal system for transporting hazardous drugs between syringes and vials doesn’t infringe two patents belonging to Baxter. U.S. District Judge Joan Lefkow granted BD summary judgment on […]
Baxter warns of alarm malfunction on some infusion pumps
Baxter (NYSE:BAX) announced today that it has issued an urgent safety communication regarding upstream occlusion alarms for certain infusion pumps. Deerfield, Illinois–based Baxter warned that incorrect administration set setup and/or the incomplete resolution of upstream occlusion alarms may result in reduced delivery or non-delivery of medication. The problem might occur without the users receiving alerts […]
Baxter touts data supporting use of machine learning for infusion pump programming
Baxter (NYSE:BAX) announced today that data shows the potential for machine learning supporting decision-making with infusion pump programming. Deerfield, Illinois-based Baxter presented the data from a retrospective study — part of a collaboration with MedAware aimed to develop next-generation dose error reduction software — at the American Society of Health-System Pharmacists (ASHP) 2021 Midyear Clinical […]