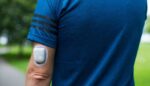
Insulet (Nasdaq:PODD) announced today that it received FDA 510(k) clearance for new enhancements to its Omnipod 5 system. The Acton, Massachusetts–based company said the updates to its tubeless, wearable automated insulin delivery patch pump’s algorithm include a new benchmark in tubeless diabetes technology with a lower, 100 mg/dL target glucose option. It also delivers a […]
Food & Drug Administration (FDA)
FDA accepts MannKind sNDA for autoinjector that treats edema
MannKind (Nasdaq:MNKD) announced that the FDA accepted a supplemental New Drug Application (sNDA) for its Furoscix ReadyFlow autoinjector. The Furoscix ReadyFlow autoinjector (SCP-111) was developed to deliver a subcutaneous furosemide injection as an investigational alternative to the FDA-approved Furoscix on-body infusion device for treating edema in adult patients with chronic heart failure (CHF) or chronic […]
Dexcom wins FDA nod for smart CGM-integrated basal dosing for type 2 diabetes
Dexcom (NSDQ:DXCM) announced today that the FDA cleared its Smart Basal CGM-integrated basal insulin dosing optimizer. The continuous glucose monitor (CGM)-based offering received clearance for adults with type 2 diabetes on glargine U-100 long-acting insulin therapy. San Diego-based Dexcom says it marks the first and only CGM-integrated basal dosing optimizer cleared for type 2 diabetes. […]
Modular Medical submits next-gen insulin pump to FDA, expects 2026 launch
Modular Medical (Nasdaq:MODD) announced that it has submitted its next-generation Pivot insulin pump to the FDA for clearance. Modular Medical’s current pump, the MODD1, won FDA clearance nearly a year ago. It has microfluidics technology that allows for the low-cost pumping of insulin. The company announced earlier this year that it began converting its manufacturing line to […]
Glucotrack now expects spring 2026 FDA IDE submission for implantable CGM
Glucotrack (Nasdaq:GCTK) today announced a slight delay in its plans to submit its long-term glucose monitor to the FDA for IDE. The company said in August that it expected a fourth-quarter investigational device exemption (IDE) submission for the long-term implantable continuous blood glucose monitor (CBGM). However, it says today that it anticipates a submission in […]
What the launch of Mobi for Android means for Tandem, people with diabetes
This week, Tandem Diabetes Care (Nasdaq:TNDM) announced a major milestone for its Mobi miniature durable insulin pump system. San Diego-based Tandem revealed that it received FDA approval for the Android version of its Mobi mobile app. Clearance brings Mobi — which the company describes as the world’s smallest, durable automated insulin delivery system — to […]
Glaukos picks up FDA approval for Epoxia incision-free keratoconus treatment
Glaukos (NYSE:GKOS) announced today that the FDA approved its Epoxia incision-free alternative to traditional corneal cross-linking procedures. Epoxia offers an advancement in corneal cross-linking for the treatment of keratoconus. Glaukos describes the rare, sight-threatening disease as “currently far too often undiagnosed and untreated.” Aliso Viejo, California-based Glaukos developed Epoxia to eliminate the need for the […]
FDA accepts application for MannKind inhaled insulin in kids
MannKind (Nasdaq:MNKD) announced today that the FDA accepted a supplemental biologics license for its Afrezza inhaled insulin. The submission, if ultimately approved, would enable MannKind to bring Afrezza to children and adolescents (aged 4-17). Afrezza is a fast-acting insulin formulation delivered through an inhaler device. MannKind engineered the mechanical inhaler device to slowly bring powder […]
GlucoSet wins FDA breakthrough nod for glucose biosensor tech
GlucoSet announced today on social media that it received FDA breakthrough device designation for its glucose monitoring technology. The company took to LinkedIn to share: “GlucoSet just received breakthrough device designation from the FDA! We’ve been pursuing this for a year and a half. Landing it puts us on the fastest possible path to U.S. […]
Biolinq wins FDA de novo nod for autonomous, needle-free CGM
Biolinq announced today that it received FDA de novo clearance for its lead product, the Biolinq Shine wearable biosensor. San Diego-based Biolinq can now scale its new generation of wearable sensors designed for comfort, simplicity and global reach. Its Shine sensor integrates glucose, activity and sleep information into a single device with autonomous operation. The […]